One-Step Synthesis of Aminobenzoic Acid Functionalized Graphene Oxide by Electrochemical Exfoliation of Graphite for Oxygen Reduction to Hydrogen Peroxide and Supercapacitors
- PMID: 36364456
- PMCID: PMC9655138
- DOI: 10.3390/molecules27217629
One-Step Synthesis of Aminobenzoic Acid Functionalized Graphene Oxide by Electrochemical Exfoliation of Graphite for Oxygen Reduction to Hydrogen Peroxide and Supercapacitors
Abstract
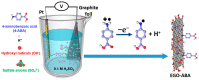
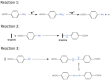
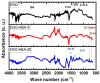
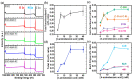
Graphene-based materials have attracted considerable attention as promising electrocatalysts for the oxygen reduction reaction (ORR) and as electrode materials for supercapacitors. In this work, electrochemical exfoliation of graphite in the presence of 4-aminebenzoic acid (4-ABA) is used as a one-step method to prepare graphene oxide materials (EGO) functionalized with aminobenzoic acid (EGO-ABA). The EGO and EGO-ABAs materials were characterized by FT-IR spectroscopy, X-ray photoelectron spectroscopy, Raman spectroscopy, X-ray diffraction and scanning electron microscopy. It was found that the EGO-ABA materials have smaller flake size and higher density of oxygenated functional groups compared to bare EGO. The electrochemical studies showed that the EGO-ABA catalysts have higher activity for the ORR to H2O2 in alkaline medium compared to EGO due to their higher density of oxygenated functional groups. However, bare EGO has a higher selectivity for the 2-electron process (81%) compared to the EGO-ABA (between 64 and 72%) which was related to a lower content of carbonyl groups. The specific capacitance of the EGO-ABA materials was higher than that of EGO, with an increase by a factor of 3 for the materials prepared from exfoliation in 5 mM 4-ABA/0.1 M H2SO4. This electrode material also showed a remarkable cycling capability with a loss of only 19.4% after 5000 cycles at 50 mVs-1.
Keywords: amine oxidation; aminobenzoic acid; electrochemical exfoliation of graphite; graphene oxide; oxygen reduction reaction; supercapacitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Li F., Jiang X., Zhao J., Zhang S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy. 2015;16:488–515. doi: 10.1016/j.nanoen.2015.07.014. - DOI
-
- Yadav R., Subhash A., Chemmenchery N., Kandasubramanian B. Graphene and Graphene Oxide for Fuel Cell Technology. Ind. Eng. Chem. Res. 2018;57:9333–9350. doi: 10.1021/acs.iecr.8b02326. - DOI
MeSH terms
Substances
Grants and funding
- STPGP 506470 - 17/Natural Sciences and Engineering Research Council
- STPGP 521437-18/Natural Sciences and Engineering Research Council
- 2014/50945-4/São Paulo Research Foundation
- 2017/10118-0/São Paulo Research Foundation
- 465571/2014-0/National Council for Scientific and Technological Development
- 303943/2021-1/National Council for Scientific and Technological Development
- Finance Code 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- Emerging Leaders in the Americas Program/EduCanada
- PhD scholarship "Énergie #286570"/Fonds de Recherche du Québec - Nature et Technologies
- team grant 3070176/Fonds de Recherche du Québec-Nature et Technologie
LinkOut - more resources
Full Text Sources

