Direct Binding of Bovine IgG-Containing Immune Complexes to Human Monocytes and Their Putative Role in Innate Immune Training
- PMID: 36364714
- PMCID: PMC9654672
- DOI: 10.3390/nu14214452
Direct Binding of Bovine IgG-Containing Immune Complexes to Human Monocytes and Their Putative Role in Innate Immune Training
Abstract
Bovine milk IgG (bIgG) was shown to bind to and neutralize the human respiratory synovial virus (RSV). In animal models, adding bIgG prevented experimental RSV infection and increased the number of activated T cells. This enhanced activation of RSV-specific T cells may be explained by receptor-mediated uptake and antigen presentation after binding of bIgG-RSV immune complexes (ICs) with FcγRs (primarily CD32) on human immune cells. This indirect effect of bIgG ICs on activation of RSV-specific T cells was confirmed previously in human T cell cultures. However, the direct binding of ICs to antigen-presenting cells has not been addressed. As bovine IgG can induce innate immune training, we hypothesized that this effect could be caused more efficiently by ICs. Therefore, we characterized the expression of CD16, CD32, and CD64 on (peripheral blood mononuclear cells (PBMCs), determined the optimal conditions to form ICs of bIgG with the RSV preF protein, and demonstrated the direct binding of these ICs to human CD14+ monocytes. Similarly, bIgG complexed with a murine anti-bIgG mAb also bound efficiently to the monocytes. To evaluate whether the ICs could induce innate immune training more efficiently than bIgG itself, the resulted ICs, as well as bIgG, were used in an in vitro innate immune training model. Training with the ICs containing bIgG and RSV preF protein-but not the bIgG alone-induced significantly higher TNF-α production upon LPS and R848 stimulation. However, the preF protein itself nonsignificantly increased cytokine production as well. This may be explained by its tropism to the insulin-like growth factor receptor 1 (IGFR1), as IGF has been reported to induce innate immune training. Even so, these data suggest a role for IgG-containing ICs in inducing innate immune training after re-exposure to pathogens. However, as ICs of bIgG with a mouse anti-bIgG mAb did not induce this effect, further research is needed to confirm the putative role of bIgG ICs in enhancing innate immune responses in vivo.
Keywords: RSV; bovine IgG; immune complex; preF protein; trained immunity.
Conflict of interest statement
R.J.J.v.N. is employed by FrieslandCampina. All other authors have nothing to declare.
Figures




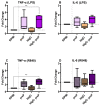
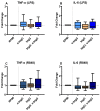
References
-
- Chen K., Chai L., Li H., Zhang Y., Xie H.-M., Shang J., Tian W., Yang P., Jiang A.C. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition. 2016;32:222–227. doi: 10.1016/j.nut.2015.08.010. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

