Machine Learning Models Predicting Cardiovascular and Renal Outcomes and Mortality in Patients with Hyperkalemia
- PMID: 36364890
- PMCID: PMC9658112
- DOI: 10.3390/nu14214614
Machine Learning Models Predicting Cardiovascular and Renal Outcomes and Mortality in Patients with Hyperkalemia
Abstract
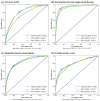
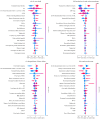
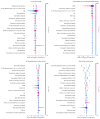
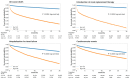
Hyperkalemia is associated with increased risks of mortality and adverse clinical outcomes. The treatment of hyperkalemia often leads to the discontinuation or restriction of beneficial but potassium-increasing therapy such as renin-angiotensin-aldosterone inhibitors (RAASi) and high-potassium diet including fruits and vegetables. To date, limited evidence is available for personalized risk evaluation in this heterogeneous and multifactorial pathophysiological condition. We developed risk prediction models using extreme gradient boosting (XGB), multiple logistic regression (LR), and deep neural network. Models were derived from a retrospective cohort of hyperkalemic patients with either heart failure or chronic kidney disease stage ≥3a from a Japanese nationwide database (1 April 2008−30 September 2018). Studied outcomes included all-cause death, renal replacement therapy introduction (RRT), hospitalization for heart failure (HHF), and cardiovascular events within three years after hyperkalemic episodes. The best performing model was further validated using an external cohort. A total of 24,949 adult hyperkalemic patients were selected for model derivation and internal validation. A total of 1452 deaths (16.6%), 887 RRT (10.1%), 1,345 HHF (15.4%), and 621 cardiovascular events (7.1%) were observed. XGB outperformed other models. The area under receiver operator characteristic curves (AUROCs) of XGB vs. LR (95% CIs) for death, RRT, HHF, and cardiovascular events were 0.823 (0.805−0.841) vs. 0.809 (0.791−0.828), 0.957 (0.947−0.967) vs. 0.947 (0.936−0.959), 0.863 (0.846−0.880) vs. 0.838 (0.820−0.856), and 0.809 (0.784−0.834) vs. 0.798 (0.772−0.823), respectively. In the external dataset including 86,279 patients, AUROCs (95% CIs) for XGB were: death, 0.747 (0.742−0.753); RRT, 0.888 (0.882−0.894); HHF, 0.673 (0.666−0.679); and cardiovascular events, 0.585 (0.578−0.591). Kaplan−Meier curves of the high-risk predicted group showed a statistically significant difference from that of the low-risk predicted groups for all outcomes (p < 0.005; log-rank test). These findings suggest possible use of machine learning models for real-world risk assessment as a guide for observation and/or treatment decision making that may potentially lead to improved outcomes in hyperkalemic patients while retaining the benefit of life-saving therapies.
Keywords: artificial intelligence; chronic kidney disease; congestive heart failure; hyperkalemia.
Conflict of interest statement
This study and the corresponding analyses were supported and funded by AstraZeneca. All authors from AstraZeneca participated in the organization of the study design, interpretation of the results, contribution to the manuscript drafts and revisions, and the decision to approve publication of the final manuscript. E.K. reports consultancy and speaker bureau fees from AstraZeneca. S.O. and T.Y. are employed by AstraZeneca. S.K. reports investigator-initiated grant funding from Bayer and Daiichi Sankyo, and personal fees from AstraZeneca, Bayer, Bristol Myers Squibb and Pfizer. M.O. and X.M. are employed by IQVIA Solutions Japan K.K. T.K. is employed by RWD Co. Ltd. which supports maintenance of the RWD database owned by the Health, Clinic and Education Information Evaluation Institute (Kyoto, Japan). IQVIA Solutions Japan K.K. did not own the database used, neither MDV nor RWD databases. Therefore, both MDV and RWD databases were not concerned with any conflicts of interest. In this study, both IQVIA Solution Japan K.K. and RWD Co. Ltd. were funded by AstraZeneca to perform their roles. The model derivation and internal validation were performed by IQVIA Solutions Japan K.K. and the external validation was performed by RWD Co., Ltd.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

