Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus
- PMID: 36364913
- PMCID: PMC9657080
- DOI: 10.3390/nu14214650
Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus
Abstract
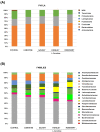
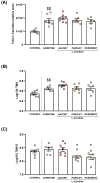
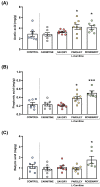
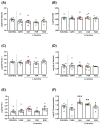
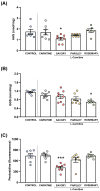
Ischemic heart disease (IHD) and type-2 diabetes mellitus (T2DM) remain major health problems worldwide and commonly coexist in individuals. Gut microbial metabolites, such as trimethylamine N-oxide (TMAO) and short-chain fatty acids (SCFAs), have been linked to cardiovascular and metabolic diseases. Previous studies have reported dysbiosis in the gut microbiota of these patients and the prebiotic effects of some components of the Mediterranean diet. Essential oil emulsions of savory (Satureja hortensis), parsley (Petroselinum crispum) and rosemary (Rosmarinus officinalis) were assessed as nutraceuticals and prebiotics in IHD and T2DM. Humanized mice harboring gut microbiota derived from that of patients with IHD and T2DM were supplemented with L-carnitine and orally treated with essential oil emulsions for 40 days. We assessed the effects on gut microbiota composition and abundance, microbial metabolites and plasma markers of cardiovascular disease, inflammation and oxidative stress. Our results showed that essential oil emulsions in mice supplemented with L-carnitine have prebiotic effects on beneficial commensal bacteria, mainly Lactobacillus genus. There was a decrease in plasma TMAO and an increase in fecal SCFAs levels in mice treated with parsley and rosemary essential oils. Thrombomodulin levels were increased in mice treated with savory and parsley essential oils. While mice treated with parsley and rosemary essential oils showed a decrease in plasma cytokines (INFɣ, TNFα, IL-12p70 and IL-22); savory essential oil was associated with increased levels of chemokines (CXCL1, CCL2 and CCL11). Finally, there was a decrease in protein carbonyls and pentosidine according to the essential oil emulsion. These results suggest that changes in the gut microbiota induced by essential oils of parsley, savory and rosemary as prebiotics could differentially regulate cardiovascular and metabolic factors, which highlights the potential of these nutraceuticals for reducing IHD risk in patients affected by T2DM.
Keywords: carbonyl; chemokine; cytokine; nutraceutical; parsley; pentosidine; prebiotic; protein carbonyl; rosemary; savory; short-chain fatty acid; trimethylamine N-oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Timmis A., Vardas P., Townsend N., Torbica A., Katus H., De Smedt D., Gale C.P., Maggioni A.P., Petersen S.E., Huculeci R., et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021: Executive Summary. Eur. Hear. J.—Qual. Care Clin. Outcomes. 2022;8:377–382. doi: 10.1093/ehjqcco/qcac014. - DOI - PubMed
-
- Sanchez-Alcoholado L., Castellano-Castillo D., Jordán-Martínez L., Moreno-Indias I., Cardila-Cruz P., Elena D., Muñoz-Garcia A.J., Queipo-Ortuño M.I., Jimenez-Navarro M. Role of Gut Microbiota on Cardio-Metabolic Parameters and Immunity in Coronary Artery Disease Patients with and without Type-2 Diabetes Mellitus. Front. Microbiol. 2017;8:1936. doi: 10.3389/fmicb.2017.01936. - DOI - PMC - PubMed

