Effect of Black Corn Anthocyanin-Rich Extract (Zea mays L.) on Cecal Microbial Populations In Vivo (Gallus gallus)
- PMID: 36364942
- PMCID: PMC9655515
- DOI: 10.3390/nu14214679
Effect of Black Corn Anthocyanin-Rich Extract (Zea mays L.) on Cecal Microbial Populations In Vivo (Gallus gallus)
Abstract
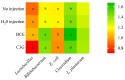
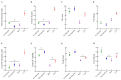
Black corn has been attracting attention to investigate its biological properties due to its anthocyanin composition, mainly cyanidin-3-glucoside. Our study evaluated the effects of black corn extract (BCE) on intestinal morphology, gene expression, and the cecal microbiome. The BCE intra-amniotic administration was evaluated by an animal model in Gallus gallus. The eggs (n = 8 per group) were divided into: (1) no injection; (2) 18 MΩ H2O; (3) 5% black corn extract (BCE); and (4) 0.38% cyanidin-3-glucoside (C3G). A total of 1 mL of each component was injected intra-amniotic on day 17 of incubation. On day 21, the animals were euthanized after hatching, and the duodenum and cecum content were collected. The cecal microbiome changes were attributed to BCE administration, increasing the population of Bifidobacterium and Clostridium, and decreasing E. coli. The BCE did not change the gene expression of intestinal inflammation and functionality. The BCE administration maintained the villi height, Paneth cell number, and goblet cell diameter (in the villi and crypt), similar to the H2O injection but smaller than the C3G. Moreover, a positive correlation was observed between Bifidobacterium, Clostridium, E. coli, and villi GC diameter. The BCE promoted positive changes in the cecum microbiome and maintained intestinal morphology and functionality.
Keywords: cyanidin; goblet cells; intestinal barrier; phenolic components.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sui X., Zhang Y., Jiang L., Zhou W. Anthocyanins in Food. Encycl. Food Chem. 2019;2:10–17.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

