How to Improve Solubility and Dissolution of Irbesartan by Fabricating Ternary Solid Dispersions: Optimization and In-Vitro Characterization
- PMID: 36365083
- PMCID: PMC9693646
- DOI: 10.3390/pharmaceutics14112264
How to Improve Solubility and Dissolution of Irbesartan by Fabricating Ternary Solid Dispersions: Optimization and In-Vitro Characterization
Abstract
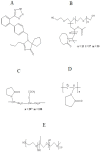
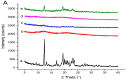
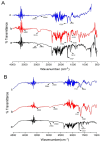
The purpose of this study is to improve the solubility and dissolution of a poorly soluble drug, Irbesartan, using solid dispersion techniques. For that purpose, different polymers such as Soluplus®, Kollidon® VA 64, Kolliphor® P 407, and Polyinylpyrrolidone (PVP-K30) were used as carriers at different concentrations to prepare solid dispersion formulations through the solvent evaporation method. In order to prepare binary dispersion formulations, Soluplus® and Kollidon® VA 64 were used at drug: polymer ratios of 1:1, 1:2, 1:3, and 1:4 (w/w). Saturation solubility of the drug in the presence of used carriers was performed to investigate the quantitative increase in solubility. Dissolution studies were performed to explore the drug release behavior from the prepared dispersions. Additionally, the characterization of the prepared formulations was carried out by performing DSC, SEM, XRD, and FTIR studies. The results revealed that among binary systems, K4 formulation (Drug: Kollidon® VA 64 at ratio of 1:4 w/w) exhibited optimal performance in terms of increased solubility, drug release, and other investigated parameters. Furthermore, ternary dispersion formulations of the optimized binary formulation were prepared with two more polymers, Kolliphor® P 407 and Polyvinylpyrrolidone (PVP-K30), at (Drug: Kollidon® VA 64:ternary polymer) ratios of 1:4:1, 1:4:2, and 1:4:3 (w/w). The results showed that KPVP (TD3) exhibited the highest increase in solubility, as well as dissolution rate, among ternary solid dispersion formulations. Results of solubility enhancement by ternary solid dispersion formulations were also supported by FTIR, DSC, XRD, and SEM analysis. Conclusively, it was found that the ternary solid dispersion-based systems were more effective compared to the binary combinations in improving solubility as well as dissolution of a poorly soluble drug (Irbesartan).
Keywords: Irbesartan; Kollidon® VA 64; Kolliphor® P 407; Polyinylpyrrolidone (PVP-K30); Soluplus®; dissolution; health care; solid dispersion; solubility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Sharma K.S., Sahoo J., Agrawal S., Kumari A. Solid dispersions: A technology for improving bioavailability. J. Anal. Pharm. Res. 2019;8:127–133. doi: 10.15406/japlr.2019.08.00326. - DOI
-
- Teodorescu M., Bercea M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Technol. Eng. 2015;54:923–943. doi: 10.1080/03602559.2014.979506. - DOI
-
- Arora R., Malhotra P., Sundriyal S., Yashavanth H.S. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease: Bioactive Foods in Chronic Disease States. Elsevier; Amsterdam, The Netherlands: 2012. Medicinal plants as remedies for gastrointestinal ailments and diseases: A review; pp. 301–311.
-
- Dhirendra K. Solid dispersions: A review. Pak. J. Pharm. Sci. 2009;22:234–246. - PubMed
-
- Khatri P., Shah M.K., Patel N., Jain S., Vora N., Lin S. Preparation and characterization of pyrimethamine solid dispersions and an evaluation of the physical nature of pyrimethamine in solid dispersions. J. Drug Deliv. Sci. Technol. 2018;45:110–123. doi: 10.1016/j.jddst.2018.03.012. - DOI
LinkOut - more resources
Full Text Sources

