Drug Stability: ICH versus Accelerated Predictive Stability Studies
- PMID: 36365143
- PMCID: PMC9693625
- DOI: 10.3390/pharmaceutics14112324
Drug Stability: ICH versus Accelerated Predictive Stability Studies
Abstract
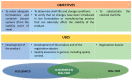
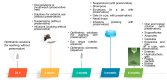
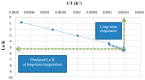
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), along with the World Health Organization (WHO), has provided a set of guidelines (ICH Q1A-E, Q3A-B, Q5C, Q6A-B) intended to unify the standards for the European Union, Japan, and the United States to facilitate the mutual acceptance of stability data that are sufficient for registration by the regulatory authorities in these jurisdictions. Overall, ICH stability studies involve a drug substance tested under storage conditions and assess its thermal stability and sensitivity to moisture. The long-term testing should be performed over a minimum of 12 months at 25 °C ± 2 °C/60% RH ± 5% RH or at 30 °C ± 2 °C/65% RH ± 5% RH. The intermediate and accelerated testing should cover a minimum of 6 months at 30 °C ± 2 °C/65% RH ± 5% RH (which is not necessary if this condition was utilized as a long-term one) and 40 °C ± 2 °C/75% RH ± 5% RH, respectively. Hence, the ICH stability testing for industrially fabricated medicines is rigorous and tedious and involves a long period of time to obtain preclinical stability data. For this reason, Accelerated Predictive Stability (APS) studies, carried out over a 3-4-week period and combining extreme temperatures and RH conditions (40-90 °C)/10-90% RH, have emerged as novel approaches to predict the long-term stability of pharmaceutical products in a more efficient and less time-consuming manner. In this work, the conventional ICH stability studies versus the APS approach will be reviewed, highlighting the advantages and disadvantages of both strategies. Furthermore, a comparison of the stability requirements for the commercialization of industrially fabricated medicines versus extemporaneous compounding formulations will be discussed.
Keywords: ASP; extemporaneous compounding; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- WHO . Annex 10. Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products. WHO; Geneva, Switzerland: 2018.
-
- USP Stability Considerations in Dispensing Practice (1191) 2020. [(accessed on 12 September 2021)]. Available online: http://www.uspbpep.com/usp32/pub/data/v32270/usp32nf27s0_c1191.html#usp3....
-
- Jato J.L.V. Tecnologia Farmaceutica Volumen I: Aspectos Fundamentales de los Sistemas Farmacéuticos y Operaciones Básicas. Editorial Sintesis; Madrid, Spain: 2001. Estabilidad; pp. 317–362.
-
- Carstensen J.T. Drug Stability, Principles and Practices. Elsevier; New York, NY, USA: 2000.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

