Membrane Vesicles Derived from Gut Microbiota and Probiotics: Cutting-Edge Therapeutic Approaches for Multidrug-Resistant Superbugs Linked to Neurological Anomalies
- PMID: 36365188
- PMCID: PMC9692612
- DOI: 10.3390/pharmaceutics14112370
Membrane Vesicles Derived from Gut Microbiota and Probiotics: Cutting-Edge Therapeutic Approaches for Multidrug-Resistant Superbugs Linked to Neurological Anomalies
Abstract
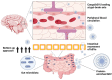
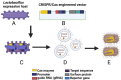
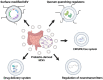
Multidrug-resistant (MDR) superbugs can breach the blood-brain barrier (BBB), leading to a continuous barrage of pro-inflammatory modulators and induction of severe infection-related pathologies, including meningitis and brain abscess. Both broad-spectrum or species-specific antibiotics (β-lactamase inhibitors, polymyxins, vancomycin, meropenem, plazomicin, and sarecycline) and biocompatible poly (lactic-co-glycolic acid) (PLGA) nanoparticles have been used to treat these infections. However, new therapeutic platforms with a broad impact that do not exert off-target deleterious effects are needed. Membrane vesicles or extracellular vesicles (EVs) are lipid bilayer-enclosed particles with therapeutic potential owing to their ability to circumvent BBB constraints. Bacteria-derived EVs (bEVs) from gut microbiota are efficient transporters that can penetrate the central nervous system. In fact, bEVs can be remodeled via surface modification and CRISPR/Cas editing and, thus, represent a novel platform for conferring protection against infections breaching the BBB. Here, we discuss the latest scientific research related to gut microbiota- and probiotic-derived bEVs, and their therapeutic modifications, in terms of regulating neurotransmitters and inhibiting quorum sensing, for the treatment of neurodegenerative diseases, such as Parkinson's and Alzheimer's diseases. We also emphasize the benefits of probiotic-derived bEVs to human health and propose a novel direction for the development of innovative heterologous expression systems to combat BBB-crossing pathogens.
Keywords: blood–brain barrier; extracellular vesicles; gut microbiota; membrane vesicles; meningitis; probiotics; superbugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Bacterial Extracellular Vesicles and the Gut-Microbiota Brain Axis: Emerging Roles in Communication and Potential as Therapeutics.Adv Biol (Weinh). 2021 Jul;5(7):e2000540. doi: 10.1002/adbi.202000540. Epub 2021 Apr 15. Adv Biol (Weinh). 2021. PMID: 33857347 Review.
-
Peripheral extracellular vesicles in neurodegeneration: pathogenic influencers and therapeutic vehicles.J Nanobiotechnology. 2024 Apr 12;22(1):170. doi: 10.1186/s12951-024-02428-1. J Nanobiotechnology. 2024. PMID: 38610012 Free PMC article. Review.
-
Extracellular vesicles as a novel mediator of interkingdom communication.Cytokine Growth Factor Rev. 2023 Oct;73:173-184. doi: 10.1016/j.cytogfr.2023.08.005. Epub 2023 Aug 21. Cytokine Growth Factor Rev. 2023. PMID: 37634980 Review.
-
Bacterial extracellular vesicles in the initiation, progression and treatment of atherosclerosis.Gut Microbes. 2025 Dec;17(1):2452229. doi: 10.1080/19490976.2025.2452229. Epub 2025 Jan 22. Gut Microbes. 2025. PMID: 39840620 Review.
-
Diet, commensal microbiota-derived extracellular vesicles, and host immunity.Eur J Immunol. 2023 Jul;53(7):e2250163. doi: 10.1002/eji.202250163. Epub 2023 May 17. Eur J Immunol. 2023. PMID: 37137164 Review.
Cited by
-
Membrane Vesicles as Drug Delivery Systems: Source, Preparation, Modification, Drug Loading, In Vivo Administration and Biodistribution, and Application in Various Diseases.Pharmaceutics. 2023 Jul 7;15(7):1903. doi: 10.3390/pharmaceutics15071903. Pharmaceutics. 2023. PMID: 37514089 Free PMC article.
-
Bio-Inspired Drug Delivery Systems: From Synthetic Polypeptide Vesicles to Outer Membrane Vesicles.Pharmaceutics. 2023 Jan 21;15(2):368. doi: 10.3390/pharmaceutics15020368. Pharmaceutics. 2023. PMID: 36839691 Free PMC article. Review.
-
Effect of gut microbiota-derived metabolites and extracellular vesicles on neurodegenerative disease in a gut-brain axis chip.Nano Converg. 2024 Feb 10;11(1):7. doi: 10.1186/s40580-024-00413-w. Nano Converg. 2024. PMID: 38340254 Free PMC article.
-
Extracellular Vesicles of Probiotics: Shedding Light on the Biological Activity and Future Applications.Pharmaceutics. 2023 Feb 4;15(2):522. doi: 10.3390/pharmaceutics15020522. Pharmaceutics. 2023. PMID: 36839844 Free PMC article. Review.
-
Gram-negative bacterial sRNAs encapsulated in OMVs: an emerging class of therapeutic targets in diseases.Front Cell Infect Microbiol. 2024 Jan 30;13:1305510. doi: 10.3389/fcimb.2023.1305510. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38983695 Free PMC article. Review.
References
-
- Muldoon L.L., Alvarez J.I., Begley D.J., Boado R.J., Del Zoppo G.J., Doolittle N.D., Engelhardt B., Hallenbeck J.M., Lonser R.R., Ohlfest J.R., et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J. Cereb. Blood Flow Metab. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

