Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells
- PMID: 36365221
- PMCID: PMC9699314
- DOI: 10.3390/pharmaceutics14112403
Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells
Abstract
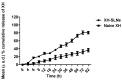
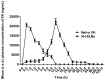
Many natural products with greater therapeutic efficacy are limited to target several chronic diseases such as cancer, diabetes, and neurodegenerative diseases. Among the natural products from hops, i.e., Xanthohumol (XH), is a prenylated chalcone. The present research work focuses on the enhancement of the poor oral bioavailability and weak pharmacokinetic profile of XH. We exemplified the development of a Xanthohumol-loaded solid lipid nanoparticles (XH-SLNs) cargo system to overcome the limitations associated with its bioavailability. The XH-SLNs were prepared by a high-shear homogenization/ultrasonication method and graphical, numerical optimization was performed by using Box-Behnken Design. Optimized XH-SLNs showed PS (108.60 nm), PDI (0.22), ZP (-12.70 mV), %EE (80.20%) and an amorphous nature that was confirmed by DSC and PXRD. FE-SEM and HRTEM revealed the spherical morphology of XH-SLNs. The results of release studies were found to be 9.40% in 12 h for naive XH, whereas only 28.42% of XH was released from XH-SLNs. The slow release of drugs may be due to immobilization of XH in the lipid matrix. In vivo pharmacokinetic study was performed for the developed XH-SLNs to verify the enhancement in the bioavailability of XH than naive XH. The enhancement in the bioavailability of the XH was confirmed from an increase in Cmax (1.07-folds), AUC0-t (4.70-folds), t1/2 (6.47-folds) and MRT (6.13-folds) after loading into SLNs. The relative bioavailability of XH loaded in SLNs and naive XH was found to be 4791% and 20.80%, respectively. The cytotoxicity study of naive XH, XH-SLNs were performed using PC-3 cell lines by taking camptothecin as positive control. The results of cytotoxicity study revealed that XH-SLNs showed good cell inhibition in a sustained pattern. This work successfully demonstrated formulation of XH-SLNs with sustained release profile and improved oral bioavailability of XH with good anticancer properties against PC-3 cells.
Keywords: Box–Behnken Design; Xanthohumol; drug release; oral bioavailability; solid lipid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Verzele M., Stockx J., Fontijn F., Anteunis M. Xanthohumol, a New Natural Chalkone. Bull. Soc. Chim. Belg. 1957;66:452–475. doi: 10.1002/bscb.19570660137. - DOI
-
- Parvez S., Yadagiri G., Gedda M.R., Singh A., Singh O.P., Verma A., Sundar S., Mudavath S.L. Modified Solid Lipid Nanoparticles Encapsulated with Amphotericin B and Paromomycin: An Effective Oral Combination against Experimental Murine Visceral Leishmaniasis. Sci. Rep. 2020;10:12243. doi: 10.1038/s41598-020-69276-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

