Cyclodextrins and Their Polymers Affect the Lipid Membrane Permeability and Increase Levofloxacin's Antibacterial Activity In Vitro
- PMID: 36365470
- PMCID: PMC9654586
- DOI: 10.3390/polym14214476
Cyclodextrins and Their Polymers Affect the Lipid Membrane Permeability and Increase Levofloxacin's Antibacterial Activity In Vitro
Abstract
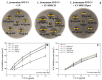
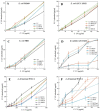
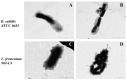
Cyclodextrins (CDs) are promising drug carriers that are used in medicine. We chose CDs with different substituents (polar/apolar, charged/neutral) to obtain polymers (CDpols) with different properties. CDpols are urethanes with average Mw of ~120 kDa; they form nanoparticles 100-150 nm in diameter with variable ζ-potential. We studied the interaction of CD and CDpols with model (liposomal) and bacterial membranes. Both types of CD carriers cause an increase in the liposomal membrane permeability, and for polymers, this effect was almost two times stronger. The formation of CD/CDpols complexes with levofloxacin (LV) enhances LV's antibacterial action 2-fold in vitro on five bacterial strains. The most pronounced effect was determined for LV-CD complexes. LV-CDs and LV-CDpols adsorb on bacteria, and cell morphology influences this process dramatically. According to TEM studies, the rough surface and proteinaceous fimbria of Gram-negative E. coli facilitate the adsorption of CD particles, whereas the smooth surface of Gram-positive bacteria impedes it. In comparison with LV-CDs, LV-CDpols are adsorbed 15% more effectively by E. coli, 2.3-fold better by lactobacilli and 5-fold better in the case of B. subtilis. CDs and CDpols are not toxic for bacterial cells, but may cause mild defects that, in addition to LV-CD carrier adsorption, improve LV's antibacterial properties.
Keywords: antibacterial activity; cyclodextrin; levofloxacin; liposome; spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Dua K., Rapalli V.K., Shukla S.D., Singhvi G., Shastri M.D., Chellappan D.K., Satija S., Mehta M., Gulati M., Pinto T.D.J.A., et al. Multi-drug resistant Mycobacterium tuberculosis & oxidative stress complexity: Emerging need for novel drug delivery approaches. Biomed. Pharmacother. 2018;107:1218–1229. doi: 10.1016/j.biopha.2018.08.101. - DOI - PubMed
-
- Řezanka M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2019;17:49–63. doi: 10.1007/s10311-018-0779-7. - DOI
-
- Kicuntod J., Sangpheak K., Mueller M., Wolschann P., Viernstein H., Yanaka S., Kato K., Chavasiri W., Pongsawasdi P., Kungwan N., et al. Theoretical and experimental studies on inclusion complexes of pinostrobin and β-cyclodextrins. Sci. Pharm. 2018;86:5. doi: 10.3390/scipharm86010005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

