Solvent Evaporation Rate as a Tool for Tuning the Performance of a Solid Polymer Electrolyte Gas Sensor
- PMID: 36365751
- PMCID: PMC9658810
- DOI: 10.3390/polym14214758
Solvent Evaporation Rate as a Tool for Tuning the Performance of a Solid Polymer Electrolyte Gas Sensor
Abstract
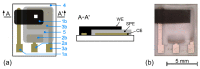
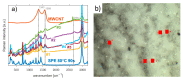
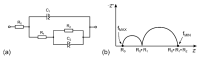
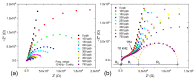
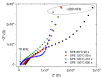
Solid polymer electrolytes show their potential to partially replace conventional electrolytes in electrochemical devices. The solvent evaporation rate represents one of many options for modifying the electrode-electrolyte interface by affecting the structural and electrical properties of polymer electrolytes used in batteries. This paper evaluates the effect of solvent evaporation during the preparation of solid polymer electrolytes on the overall performance of an amperometric gas sensor. A mixture of the polymer host, solvent and an ionic liquid was thermally treated under different evaporation rates to prepare four polymer electrolytes. A carbon nanotube-based working electrode deposited by spray-coating the polymer electrolyte layer allowed the preparation of the electrode-electrolyte interface with different morphologies, which were then investigated using scanning electron microscopy and Raman spectroscopy. All prepared sensors were exposed to nitrogen dioxide concentration of 0-10 ppm, and the current responses and their fluctuations were analyzed. Electrochemical impedance spectroscopy was used to describe the sensor with an equivalent electric circuit. Experimental results showed that a higher solvent evaporation rate leads to lower sensor sensitivity, affects associated parameters (such as the detection/quantification limit) and increases the limit of the maximum current flowing through the sensor, while the other properties (hysteresis, repeatability, response time, recovery time) change insignificantly.
Keywords: gas sensor; ionic liquid; noise spectroscopy; solid polymer electrolyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ye Y.-S., Rick J., Hwang B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A. 2013;1:2719–2743. doi: 10.1039/C2TA00126H. - DOI
-
- Correia D.M., Fernandes L.C., Martins P.M., García-Astrain C., Costa C.M., Reguera J., Lanceros-Méndez S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020;30:1909736. doi: 10.1002/adfm.201909736. - DOI
-
- Josef E., Yan Y., Stan M.C., Wellmann J., Vizintin A., Winter M., Johansson P., Dominko R., Guterman R. Ionic Liquids and their Polymers in Lithium-Sulfur Batteries. Isr. J. Chem. 2019;59:832–842. doi: 10.1002/ijch.201800159. - DOI
-
- Austin Suthanthiraraj S., Johnsi M. Nanocomposite polymer electrolytes. Ionics (Kiel) 2017;23:2531–2542. doi: 10.1007/s11581-016-1924-6. - DOI
-
- Xia W., Zhang Z. PVDF-based dielectric polymers and their applications in electronic materials. IET Nanodielectrics. 2018;1:17–31. doi: 10.1049/iet-nde.2018.0001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

