Evaluation of the Efficacy of COVID-19 Booster Vaccinations in Healthcare Personnel
- PMID: 36366305
- PMCID: PMC9698918
- DOI: 10.3390/vaccines10111797
Evaluation of the Efficacy of COVID-19 Booster Vaccinations in Healthcare Personnel
Abstract
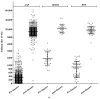
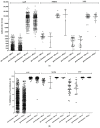
This study aimed to investigate the efficacy of different COVID-19 booster vaccines by measuring the serum antibody titer. SARS-CoV-2 anti-nucleocapsid protein antibody (N-Ab), anti-spike protein antibody (S-Ab), and neutralizing antibody (Neut.Ab) were measured before and 4-6 weeks after booster vaccinations in healthcare personnel with a previous vaccination within 3-6 months. Personnel who previously received two doses of ChAdOx1 vaccine or two doses of BNT162b2 vaccine received the BNT162b2 vaccine (AAP and PPP groups, respectively). Personnel who previously received two doses of mRNA-1273 received the same vaccine as a booster dose (MMM group). Of the 917 participants, the AAP, MMM, and PPP groups comprised 837 (91.3%), 27 (2.9%), and 53 (5.8%) participants, respectively. The pre-booster S-Ab and Neut.Ab titer were significantly lower in the AAP group. After the booster vaccination, all participants were positive for S-Ab and Neut.Ab; furthermore, the S-Ab and Neut.Ab titer significantly increased in all three groups, although the post-booster S-Ab was lower in the AAP group than in the other groups. The post-booster Neut.Ab titer showed no significant difference among the groups. Our study's results suggest that booster vaccination, after two prior vaccinations, shows a significant effect regardless of the type of vaccine administered.
Keywords: COVID-19 vaccine booster shot; COVID-19 vaccines; SARS-CoV-2; antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buera F.J., Fattal-Jaef R.N., Hopenhayn H., Neumeyer P.A., Shin Y. The Economic Ripple Effects of COVID-19. National Bureau of Economic Research; Cambridge, MA, USA: 2021.
LinkOut - more resources
Full Text Sources
Miscellaneous

