Can a Two-Dose Influenza Vaccine Regimen Better Protect Older Adults? An Agent-Based Modeling Study
- PMID: 36366307
- PMCID: PMC9697266
- DOI: 10.3390/vaccines10111799
Can a Two-Dose Influenza Vaccine Regimen Better Protect Older Adults? An Agent-Based Modeling Study
Abstract
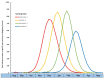
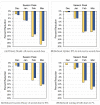
Older adults (age ≥ 65) are at high risk of influenza morbidity and mortality. This study evaluated the impact of a hypothetical two-dose influenza vaccine regimen per season to reduce symptomatic flu cases by providing preseason (first dose) and mid-season (second dose) protection to offset waning vaccine effectiveness (VE). The Framework for Reconstructing Epidemiological Dynamics (FRED), an agent-based modeling platform, was used to compare typical one-dose vaccination to a two-dose vaccination strategy. Primary models incorporated waning VE of 10% per month and varied influenza season timing (December through March) to estimate cases and hospitalizations in older adults. Additional scenarios modeled reductions in uptake and VE of the second dose, and overall waning. In seasons with later peaks, two vaccine doses had the largest potential to reduce cases (14.4% with February peak, 18.7% with March peak) and hospitalizations (13.1% with February peak, 16.8% with March peak). Reductions in cases and hospitalizations still resulted but decreased when 30% of individuals failed to receive a second dose, second dose VE was reduced, or overall waning was reduced to 7% per month. Agent-based modeling indicates that two influenza vaccine doses could decrease cases and hospitalizations in older individuals. The highest impact occurred in the more frequently observed late-peak seasons. The beneficial impact of the two-dose regimen persisted despite model scenarios of reduced uptake of the second dose, decreased VE of the second dose, or overall VE waning.
Keywords: adult; influenza; vaccine.
Conflict of interest statement
The authors KVW, MGK, and MGR declare no conflict of interest. RKZ has research grant funding from Sanofi Pasteur. LHH has done consulting with Sanofi Pasteur, GSK, Pfizer, and Merck without compensation. JVW serves on a Scientific Advisory Board for Quidel. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Centers for Disease Control Flu Vaccination Coverage, United States, 2019–2020 Influenza Season. [(accessed on 25 May 2021)]; Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm.
-
- Centers for Disease Control and Prevention Influenza (Flu), Adults 65 & Over. [(accessed on 27 July 2022)]; Available online: https://www.cdc.gov/flu/highrisk/65over.htm#:~:text=In%20recent%20years%....
-
- Centers for Disease Control and Prevention Influenza (Flu), When is Flu Season. [(accessed on 27 July 2022)]; Available online: https://www.cdc.gov/flu/about/season/flu-season.htm.
-
- Ferdinands J.M., Gaglani M., Martin E.T., Monto A.S., Middleton D., Silveira F., Talbot H.K., Zimmerman R., Patel M. Waning Vaccine Effectiveness Against Influenza-Associated Hospitalizations Among Adults, 2015–2016 to 2018–2019, United States Hospitalized Adult Influenza Vaccine Effectiveness Network. Clin. Infect. Dis. 2021;73:726–729. doi: 10.1093/cid/ciab045. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention Influenza Vaccination Coverage for Persons 6 Months and Older. [(accessed on 27 July 2022)]; Available online: https://www.cdc.gov/flu/fluvaxview/interactive-general-population.htm.
Grants and funding
LinkOut - more resources
Full Text Sources

