Development of Bivalent mRNA Vaccines against SARS-CoV-2 Variants
- PMID: 36366316
- PMCID: PMC9693459
- DOI: 10.3390/vaccines10111807
Development of Bivalent mRNA Vaccines against SARS-CoV-2 Variants
Abstract
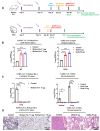
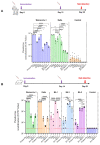
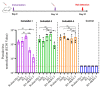
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected billions of individuals and is the cause of the current global coronavirus disease 2019 (COVID-19) pandemic. We previously developed an mRNA vaccine (LVRNA009) based on the S protein of the Wuhan-Hu-1 strain; the phases I and II clinical trials showed that LVRNA009 has a promising safety and immunogenicity profile. In order to counteract the immune escape by SARS-CoV-2 variants of concern, a panel of mRNA vaccines was developed based on the S proteins of the Wuhan-Hu-1, Delta, Omicron BA.1, BA.2, and BA.5 strains, and each vaccine’s protective potency against the virus variants was evaluated. Furthermore, to achieve excellent neutralization against SARS-CoV-2 variants, bivalent vaccines were developed and tested against the variants. We found that the monovalent Wuhan-Hu-1 or the Delta vaccines could induce high level of neutralization antibody and protect animals from the infection of the SARS-CoV-2 Wuhan-Hu-1 or Delta strains, respectively. However, serum samples from mice immunized with monovalent Delta vaccine showed relatively low virus neutralization titers (VNTs) against the pseudotyped virus of the Omicron strains. Serum samples from mice immunized with bivalent Delta/BA.1 vaccine had high VNTs against the pseudotyped Wuhan-Hu-1, Delta, and BA.1 strains but low VNTs against BA.2 and BA.5 (p < 0.05). Serum samples from mice immunized with Delta/BA.2 vaccine had high VNTs against the pseudotyped Wuhan-Hu-1, Delta, BA.1 and BA.2 strains but low VNTs against BA.5. Finally, serum samples from mice immunized with Delta/BA.5 vaccine had high VNTs against all the tested pseudotyped SARS-CoV-2 strains including the Wuhan-Hu-1, Delta, and Omicron variants (p > 0.05). Therefore, a bivalent mRNA vaccine with Delta/BA.5 combination is promising to provide broad spectrum immunity against all VOCs.
Keywords: SARS-CoV-2 variants; mRNA vaccine; neutralizing antibody; spike protein.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A Beta Strain-Based Spike Glycoprotein Vaccine Candidate Induces Broad Neutralization and Protection against SARS-CoV-2 Variants of Concern.Microbiol Spectr. 2023 Feb 27;11(2):e0268722. doi: 10.1128/spectrum.02687-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36847495 Free PMC article.
-
SARS-CoV-2 bivalent mRNA vaccine with broad protection against variants of concern.Front Immunol. 2023 May 24;14:1195299. doi: 10.3389/fimmu.2023.1195299. eCollection 2023. Front Immunol. 2023. PMID: 37292197 Free PMC article.
-
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice.Nat Med. 2023 Jan;29(1):247-257. doi: 10.1038/s41591-022-02092-8. Epub 2022 Oct 20. Nat Med. 2023. PMID: 36265510 Free PMC article.
-
Development of Next Generation Vaccines against SARS-CoV-2 and Variants of Concern.Viruses. 2023 Feb 24;15(3):624. doi: 10.3390/v15030624. Viruses. 2023. PMID: 36992333 Free PMC article. Review.
-
Pseudotyped Vesicular Stomatitis Virus-Severe Acute Respiratory Syndrome-Coronavirus-2 Spike for the Study of Variants, Vaccines, and Therapeutics Against Coronavirus Disease 2019.Front Microbiol. 2022 Jan 14;12:817200. doi: 10.3389/fmicb.2021.817200. eCollection 2021. Front Microbiol. 2022. PMID: 35095820 Free PMC article. Review.
Cited by
-
Challenges in Emerging Vaccines and Future Promising Candidates against SARS-CoV-2 Variants.J Immunol Res. 2024 Jan 25;2024:9125398. doi: 10.1155/2024/9125398. eCollection 2024. J Immunol Res. 2024. PMID: 38304142 Free PMC article. Review.
-
A bivalent subunit vaccine efficiently produced in Pichia pastoris against SARS-CoV-2 and emerging variants.Front Microbiol. 2023 Jan 10;13:1093080. doi: 10.3389/fmicb.2022.1093080. eCollection 2022. Front Microbiol. 2023. PMID: 36704561 Free PMC article.
-
An mRNA-Based Respiratory Syncytial Virus Vaccine Elicits Strong Neutralizing Antibody Responses and Protects Rodents Without Vaccine-Associated Enhanced Respiratory Disease.Vaccines (Basel). 2025 Jan 9;13(1):52. doi: 10.3390/vaccines13010052. Vaccines (Basel). 2025. PMID: 39852831 Free PMC article.
-
Establishment of a Novel Platform for Developing Oral Vaccines Based on the Surface Display System of Yeast Spores.Int J Mol Sci. 2025 Apr 11;26(8):3615. doi: 10.3390/ijms26083615. Int J Mol Sci. 2025. PMID: 40332085 Free PMC article.
-
Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics.Pharmaceutics. 2025 Jul 12;17(7):903. doi: 10.3390/pharmaceutics17070903. Pharmaceutics. 2025. PMID: 40733111 Free PMC article. Review.
References
-
- Acter T., Uddin N., Das J., Akhter A., Choudhury T.R., Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020;730:138996. doi: 10.1016/j.scitotenv.2020.138996. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

