Nucleic Acid Vaccines against SARS-CoV-2
- PMID: 36366358
- PMCID: PMC9695141
- DOI: 10.3390/vaccines10111849
Nucleic Acid Vaccines against SARS-CoV-2
Abstract
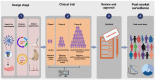
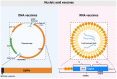
The coronavirus disease 2019 (COVID-19) has spread worldwide and imposed a substantial burden on human health, the environment, and socioeconomic development, which has also accelerated the process of nucleic acid vaccine development and licensure. Nucleic acid vaccines are viral genetic sequence-based vaccines and third-generation vaccines after whole virus vaccines and recombinant subunit vaccines, including DNA vaccines and RNA vaccines. They have many unique advantages, but there are many aspects that require optimization. Therefore, the purpose of this review is to discuss the research and development processes of nucleic acid vaccines, summarize the advantages and shortcomings, and propose further optimization strategies by taking COVID-19 vaccines as an example. Hopefully, this work can make a modest contribution in promoting the construction of emergency nucleic acid vaccine platforms and in avoiding the reemergence of similar public health emergencies.
Keywords: COVID-19; advantages and shortcomings; development process; nucleic acid vaccines; optimization.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
Publication types
Grants and funding
- 82270741/National Natural Science Foundation of China
- LY22H050001/Natural Science Foundation of Zhejiang Province
- U20A20351/National Natural Science Foundation of China
- WKJ-ZJ-2128/the Key Project of Provincial Ministry Coconstruction, Health Science, and Technology Project Plan of Zhejiang Province
- SMYY20220301001/"Yiluqihang · Shenmingyuanyang" Medical Development and Scientific Research Fund Project on Kidney Diseases
LinkOut - more resources
Full Text Sources
Miscellaneous

