Seroprevalence of Measles Antibodies in a Highly MMR-Vaccinated Population
- PMID: 36366367
- PMCID: PMC9698789
- DOI: 10.3390/vaccines10111859
Seroprevalence of Measles Antibodies in a Highly MMR-Vaccinated Population
Abstract
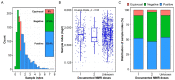
As an extremely contagious pathogen, a high rate of vaccine coverage and the durability of vaccine-induced immunity are key factors to control and eliminate measles. Herein, we assessed the seroprevalence of antibodies specific to measles in a cohort of 1393 adults (20-44 years old). ELISA results showed a nontrivial proportion of 37.6% study subjects being negative for measles immunoglobulin G (IgG). We also found significant influences of sex and age of the study cohort on the IgG level. Our findings suggest that even within a highly vaccinated population, a subset of individuals may still have sub-optimal immunity against measles and potentially be susceptible during any future measles outbreaks.
Keywords: MMR vaccine; measles virus; seroprevalence; serosurveillance; waning immunity.
Conflict of interest statement
Poland offers consultative advice to Johnson & Johnson/Janssen Global Services LLC, and is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Poland also offers consultative advice on vaccine development to Merck & Co (Keniworth, NJ, USA)., Medicago (Quebec City, Quebec, Canada), GlaxoSmithKline (Brentford, United Kingdom), Sanofi Pasteur (Lyon, France), Emergent Biosolutions (Gaithersburg, MD, USA), Dynavax (Emeryville, CA), Genentech (South San Francisco, CA, USA), Eli Lilly and Company (Indianapolis, IN, USA), Kentucky Bioprocessing Inc. (Owensboro, KY, USA), Bavarian Nordic (Hellerup, Denmark), AstraZeneca (Cambridge, United Kingdom), Exelixis (Alameda, CA, USA), Regeneron (Tarrytown, NY, USA), Janssen (Beerse, Belgium), Vyriad (Rochester, MN, USA), Moderna (Cambridge, MA, USA), and Genevant Sciences, Inc. (Cambridge, MA, USA). Poland and Ovsyannikova hold patents related to vaccinia and measles peptide vaccines. Drs. Kennedy, Poland, and Ovsyannikova hold a patent related to vaccinia peptide vaccines. Poland, Kennedy, and Ovsyannikova have received grant funding from ICW Ventures for preclinical studies on a peptide-based COVID-19 vaccine. Kennedy has received funding from Merck Research Laboratories to study waning immunity to mumps vaccine. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policy.
Figures

References
-
- Patel M., Lee A.D., Clemmons N.S., Redd S.B., Poser S., Blog D., Zucker J.R., Leung J., Link-Gelles R., Pham H., et al. National Update on Measles Cases and Outbreaks—United States, January 1–October 1, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019;68:893–896. doi: 10.15585/mmwr.mm6840e2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

