Updating the National Antigen Bank in Korea: Protective Efficacy of Synthetic Vaccine Candidates against H5Nx Highly Pathogenic Avian Influenza Viruses Belonging to Clades 2.3.2.1 and 2.3.4.4
- PMID: 36366368
- PMCID: PMC9697692
- DOI: 10.3390/vaccines10111860
Updating the National Antigen Bank in Korea: Protective Efficacy of Synthetic Vaccine Candidates against H5Nx Highly Pathogenic Avian Influenza Viruses Belonging to Clades 2.3.2.1 and 2.3.4.4
Abstract
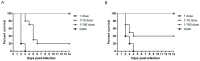
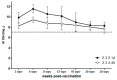
Since 2018, Korea has been building an avian influenza (AI) national antigen bank for emergency preparedness; this antigen bank is updated every 2 years. To update the vaccine strains in the antigen bank, we used reverse genetics technology to develop two vaccine candidates against avian influenza strains belonging to clades 2.3.2.1d and 2.3.4.4h, and then evaluated their immunogenicity and protective efficacy in SPF chickens challenged with H5 viruses. The two vaccine candidates, named rgCA2/2.3.2.1d and rgES3/2.3.4.4h, were highly immunogenic, with hemagglutination inhibition (HI) titers of 8.2−9.3 log2 against the vaccine strain, and 7.1−7.3 log2 against the lethal challenge viruses (in which the HA genes shared 97% and 95.4% homology with that of rgCA2/2.3.2.1d and rgES3/2.3.4.4h, respectively). A full dose of each vaccine candidate provided 100% protection against the challenge viruses, with a reduction in clinical symptoms and virus shedding. A 1/10 dose provided similar levels of protection, whereas a 1/100 dose resulted in mortality and virus shedding by 7 dpi. Moreover, immunity induced by the two vaccines was long lasting, with HI titers of >7 log2 against the vaccine strain remaining after 6 months. Thus, the two vaccine candidates show protective efficacy and can be used to update the AI national antigen bank.
Keywords: SPF chicken; antigen bank; high pathogenic avian influenza; synthesis of HA; vaccine candidates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lee C.-W., Suarez D.L., Tumpey T.M., Sung H.-W., Kwon Y.-K., Lee Y.-J., Choi J.-G., Joh S.-J., Kim M.-C., Lee E.-K., et al. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J. Virol. 2005;79:3692–3702. doi: 10.1128/JVI.79.6.3692-3702.2005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

