Immunogenicity and Durability of Antibody Responses to Homologous and Heterologous Vaccinations with BNT162b2 and ChAdOx1 Vaccines for COVID-19
- PMID: 36366372
- PMCID: PMC9692595
- DOI: 10.3390/vaccines10111864
Immunogenicity and Durability of Antibody Responses to Homologous and Heterologous Vaccinations with BNT162b2 and ChAdOx1 Vaccines for COVID-19
Abstract
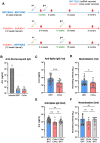
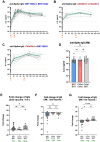
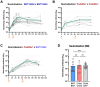
During the COVID-19 pandemic, vaccines were developed based on various platform technologies and were approved for emergency use. However, the comparative analysis of immunogenicity and durability of vaccine-induced antibody responses depending on vaccine platforms or vaccination regimens has not been thoroughly examined for mRNA- or viral vector-based vaccines. In this study, we assessed spike-binding IgG levels and neutralizing capacity in 66 vaccinated individuals prime-boost immunized either by homologous (BNT162b2-BNT162b2 or ChAdOx1-ChAdOx1) or heterologous (ChAdOx1-BNT162b2) vaccination for six months after the first vaccination. Despite the discrepancy in intervals for the prime-boost vaccination regimen of different COVID-19 vaccines, we found stronger induction and relatively rapid waning of antibody responses by homologous vaccination of the mRNA vaccine, while weaker boost effect and stable maintenance of humoral immune responses were observed in the viral vector vaccine group over 6 months. Heterologous vaccination with ChAdOx1 and BNT162b2 resulted in an effective boost effect with the highest remaining antibody responses at six months post-primary vaccination.
Keywords: BNT162b2; COVID-19; ChAdOx1; SARS-CoV-2; antibody; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. [(accessed on 11 March 2020)]. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

