Investigation of Adverse Events Experienced by Healthcare Workers following Immunization with Homologous orHeterologous COVID-19 Booster Vaccinations
- PMID: 36366377
- PMCID: PMC9696033
- DOI: 10.3390/vaccines10111869
Investigation of Adverse Events Experienced by Healthcare Workers following Immunization with Homologous orHeterologous COVID-19 Booster Vaccinations
Abstract
Objective: A comparative analysis was performed to investigate the potential risk factors of Adverse Events Following Immunization (AEFI) after receiving different booster vaccines.
Methods: From 18 January 2021 to 21 January 2022, the Health Care Workers (HCWs) of Guizhou Provincial Staff Hospital (Guizhou Province, China) who received a third Booster vaccine, that was either homologous (i.e., (i) a total of three doses of Vero cell vaccine or (ii) three doses of CHO cell vaccine) or (iii) heterologous with two first doses of Vero cell vaccine, being either CHO cell vaccine or adenovirus type-5 (Ad5) vectored COVID-19 vaccine, were asked to complete a self-report questionnaire form to provide information on any AEFI that may have occurred in the first 3 days after vaccination with the booster. The frequency of AEFI corresponding to the three different booster vaccines was compared, and the risk factors for predicting AEFI were determined by multivariate logistic regression analysis.
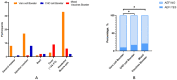
Results: Of the 904 HCWs who completed the survey, 792 met the inclusion criteria. The rates of AEFI were 9.8% (62/635) in the homologous Vero cell booster group, 17.3% (13/75) in the homologous CHO cell booster group, and 20.7% (17/82) in the heterologous mixed vaccines booster group, and the rates were significantly different (c2 = 11.5, p = 0.004) between the three groups of vaccines. Multivariate logistic regression analysis showed that: (1) compared to the homologous Vero cell booster group, the risk of AEFI was about 2.1 times higher (OR = 2.095, 95% CI: 1.056-4.157, p = 0.034) in the CHO cell booster group and 2.5 times higher (OR = 2.476, 95% CI: 1.352-4.533, p = 0.003) in the mixed vaccines group; (2) the odds for women experiencing AEFI were about 2.8 times higher (OR = 2.792, 95% CI: 1.407-5.543, p = 0.003) than men; and (3) compared to the non-frontline HCWs, the risk of AEFI was about 2.6 times higher (OR = 2.648, 95% CI: 1.473-4.760, p = 0.001) in the doctors.
Conclusion: The AEFI in all three booster groups are acceptable, and serious adverse events are rare. The risk of AEFI was higher in doctors, which may be related to the high stress during the COVID-19 epidemic. Support from government and non-governmental agencies is important for ensuring the physical and mental health of HCWs.
Keywords: adverse events following immunization (AEFI); booster COVID-19 vaccination; heterologous; homologous.
Conflict of interest statement
The authors declare no conflict of interest associated with this manuscript.
Figures
References
-
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. - DOI - PMC - PubMed
-
- Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. - DOI - PMC - PubMed
-
- Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., Sabo R.T., Hall N., Foreman A., Schubert P.L., et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1059–1062. doi: 10.15585/mmwr.mm7031e2. - DOI - PMC - PubMed
Grants and funding
- Guizhou Provincial Science and Technology Projects, Qian Ke He Foundation-ZK [2022] General 253/Yaying Li
- Doctor Foundation of Guizhou Provincial People's Hospital (GZSYBS [2019]02)/Yaying Li
- Science and Technology Fund Projects of Guizhou Health Commission (gzwkj2023-210)/Yaying Li
- Special Science and Technology Projects of Guiyang Science and Technology Bureau, Zhu Ke Contract [2022]04/Lin Liu
LinkOut - more resources
Full Text Sources
Miscellaneous