Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus
- PMID: 36366425
- PMCID: PMC9693100
- DOI: 10.3390/v14112327
Pre-Existing HSV-1 Immunity Enhances Anticancer Efficacy of a Novel Immune-Stimulating Oncolytic Virus
Abstract
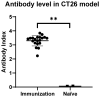
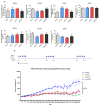
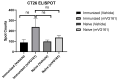
Oncolytic viruses (OVs) can specifically replicate in the host and cause cancer cell lysis while inducing an antitumor immune response. The aim of this study is to investigate the impact of either pre-existing immunity against herpes simplex virus type-1 (HSV-1) or multicycle treatment with OVs on anticancer efficacy of VG161, an HSV-1 OV in phase 2 clinical trial. VG161 efficacy was tested in CT26 mouse models by comparing the efficacy and immune response in naïve mice or in mice that were immunized with VG161. Moreover, VG161 efficacy in HLA-matched CD34+ humanized intrahepatic cholangiocarcinoma (ICC) patient-derived xenograft (PDX) models was also tested in multicycle treatment and was compared to standard chemotherapy for this type of cancer (gemcitabine). The HSV-1-immunized mice significantly inhibited tumor growth in VG161-treated mice compared to control naïve treated mice. RNA expression profiling and ELISPOT analyses indicated changes in the tumor's immune profile in the immunized and treated group compared to naïve and treated mice, as well as enhanced T cell function depicted by higher numbers of tumor specific lymphocytes, which was enhanced by immunization. In the ICC PDX model, repeated treatment of VG161 with 2 or 3 cycles seemed to increase the anticancer efficacy of VG161. In conclusion, the anticancer efficacy of VG161 can be enhanced by pre-immunization with HSV-1 and multicycle administration when the virus is given intratumorally, indicating that pre-existing antiviral immunity might enhance OV-induced antitumor immunity. Our results suggest potential clinical benefits of HSV-1-based OV therapy in HSV-1-seropositive patients and multicycle administration of VG161 for long-term maintenance treatment.
Keywords: HSV-1; immunotherapy; oncolytic viruses.
Conflict of interest statement
J.D., Y.M., Y.S., I.-F.L., I.S., X.L., W.J. and R.Z. are either current or former employees of Virogin Biotech, a company focusing on developing oncolytic virotherapy.
Figures




References
-
- Hu J.C., Coffin R.S., Davis C.J., Graham N.J., Groves N., Guest P.J., Harrington K.J., James N.D., Love C.A., McNeish I., et al. A Phase I Study of OncoVEXGM-CSF, a Second-Generation Oncolytic Herpes Simplex Virus Expressing Granulocyte Macrophage Colony-Stimulating Factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

