Sustained Virologic Suppression Reduces HIV-1 DNA Proviral Levels and HIV Antibodies in Perinatally HIV-Infected Children Followed from Birth
- PMID: 36366448
- PMCID: PMC9693172
- DOI: 10.3390/v14112350
Sustained Virologic Suppression Reduces HIV-1 DNA Proviral Levels and HIV Antibodies in Perinatally HIV-Infected Children Followed from Birth
Abstract
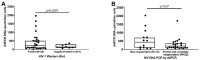
The extent to which perinatally HIV-infected children, following cART initiation, develop a low proviral reservoir burden over time, as measured by HIV DNA droplet-digital polymerase chain reaction (ddPCR) and the effect on HIV antibody is not well characterized. We measured proviral HIV DNA and plasma RNA virus load (VL) in 37 perinatally HIV-infected children at 6 months of age who initiated stable cART. At 6-11 years of age, HIV proviral DNA, HIV VL (RNA), and HIV antibody by Western Blot (WB) were assessed. CART was initiated before 6 months of age in 13 children and after 6 months in 24. At school age, the HIV DNA levels did not differ by the timing of cART, and the HIV DNA levels were lower in children with negative/indeterminate WB (p = 0.0256). Children with undetectable HIV RNA VL > 50% of the time since cART initiation had lower median DNA VL than children with undetectable VL < 50% of the time (p = 0.07). Long-term viral suppression in perinatally HIV-infected children is associated with a decrease in HIV antibodies and reduced HIV reservoirs.
Keywords: HIV DNA droplet digital PCR; early HIV treatment; perinatal HIV infection; viral load kinetics; viral reservoir burden.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- UNAIDS . Confronting Inequalities: Lessons for Pandemic Responses from 40 Years of AIDS. UNAIDS; Geneva, Switzerland: 2021.
-
- McManus M., Henderson J., Gautam A., Brody R., Weiss E.R., Persaud D., Mick E., Luzuriaga K., Investigators P. Quantitative Human Immunodeficiency Virus (HIV)-1 Antibodies Correlate with Plasma HIV-1 RNA and Cell-associated DNA Levels in Children on Antiretroviral Therapy. Clin. Infect. Dis. 2018;68:1725–1732. doi: 10.1093/cid/ciy753. - DOI - PMC - PubMed
-
- Rocca S., Zangari P., Cotugno N., De Rossi A., Ferns B., Petricone D., Rinaldi S., Giaquinto C., Bernardi S., Rojo P., et al. Human Immunodeficiency Virus (HIV)-Antibody Repertoire Estimates Reservoir Size and Time of Antiretroviral Therapy Initiation in Virally Suppressed Perinatally HIV-Infected Children. J. Pediatr. Infect. Dis. Soc. 2018;8:433–438. doi: 10.1093/jpids/piy080. - DOI - PMC - PubMed
-
- Veldsman K.A., Laughton B., Janse van Rensburg A., Zuidewind P., Dobbels E., Barnabas S., Fry S., Cotton M.F., van Zyl G.U. Viral suppression is associated with HIV-antibody level and HIV-1 DNA detectability in early treated children at 2 years of age. AIDS. 2021;35:1247–1252. doi: 10.1097/QAD.0000000000002861. - DOI - PMC - PubMed

