Geographic Range Overlap Rather than Phylogenetic Distance Explains Rabies Virus Transmission among Closely Related Bat Species
- PMID: 36366496
- PMCID: PMC9697534
- DOI: 10.3390/v14112399
Geographic Range Overlap Rather than Phylogenetic Distance Explains Rabies Virus Transmission among Closely Related Bat Species
Abstract
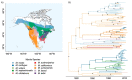
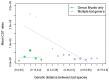
The cross-species transmission (CST) of pathogens can have dramatic consequences, as highlighted by recent disease emergence events affecting human, animal and plant health. Understanding the ecological and evolutionary factors that increase the likelihood of disease agents infecting and establishing in a novel host is therefore an important research area. Previous work across different pathogens, including rabies virus (RABV), found that increased evolutionary distance between hosts reduces the frequency of cross-species transmission and of permanent host shifts. However, whether this effect of host relatedness still holds for transmission among recently diverged hosts is not well understood. We aimed to ask if high host relatedness can still increase the probability of a host shift between more recently diverged hosts, and the importance of this effect relative to ecological predictors. We first addressed this question by quantifying the CST frequency of RABV between North American bat species within the genus Myotis, using a multi-decade data set containing 128 nucleoprotein (N) RABV sequences from ten host species. We compared RABV CST frequency within Myotis to the rates of CST between nine genera of North American bat species. We then examined whether host relatedness or host range overlap better explains the frequency of CST seen between Myotis species. We found that at the within genus scale, host range overlap, rather than host relatedness best explains the frequency of CST events. Moreover, we found evidence of CST occurring among a higher proportion of species, and CST more frequently resulting in sustained transmission in the novel host in the Myotis dataset compared to the multi-genus dataset. Our results suggest that among recently diverged species, the ability to infect a novel host is no longer restricted by physiological barriers but instead is limited by physical contact. Our results improve predictions of where future CST events for RABV might occur and clarify the relationship between host divergence and pathogen emergence.
Keywords: Myotis bat; North America; cross-species transmission; genetic divergence; host shifts; host-pathogen interaction; niche overlap; rabies; range overlap.
Conflict of interest statement
The authors declare having no conflict of interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

