Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection
- PMID: 36366514
- PMCID: PMC9693925
- DOI: 10.3390/v14112417
Use of Human Lung Tissue Models for Screening of Drugs against SARS-CoV-2 Infection
Abstract
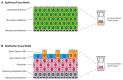
The repurposing of licenced drugs for use against COVID-19 is one of the most rapid ways to develop new and alternative therapeutic options to manage the ongoing pandemic. Given circa 7817 licenced compounds available from Compounds Australia that can be screened, this paper demonstrates the utility of commercially available ex vivo/3D airway and alveolar tissue models. These models are a closer representation of in vivo studies than in vitro models, but retain the benefits of rapid in vitro screening for drug efficacy. We demonstrate that several existing drugs appear to show anti-SARS-CoV-2 activity against both SARS-CoV-2 Delta and Omicron Variants of Concern in the airway model. In particular, fluvoxamine, as well as aprepitant, everolimus, and sirolimus, has virus reduction efficacy comparable to the current standard of care (remdesivir, molnupiravir, nirmatrelvir). Whilst these results are encouraging, further testing and efficacy studies are required before clinical use can be considered.
Keywords: 3D tissue models; COVID-19; CoviRx.org; drug repurposing; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Worldometers.Info COVID-19 Coronavirus Pandemic. 2022. [(accessed on 18 September 2022)]. Available online: https://www.worldometers.info/coronavirus/
-
- Wang H., Paulson K.R., Pease S.A., Watson S., Comfort H., Zheng P., Aravkin A.Y., Bisignano C., Barber R.M., Alam T., et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

