Pathogenicity and Transmissibility of Goose-Origin H5N6 Avian Influenza Virus Clade 2.3.4.4h in Mammals
- PMID: 36366552
- PMCID: PMC9699601
- DOI: 10.3390/v14112454
Pathogenicity and Transmissibility of Goose-Origin H5N6 Avian Influenza Virus Clade 2.3.4.4h in Mammals
Abstract
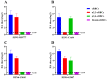
Throughout the last decade, H5N6 avian influenza viruses (AIVs) circulating in poultry and infecting humans have caused increasing global concerns that they might become a pandemic threat to global health. Since AIVs could occasionally cause asymptomatic infections in geese, virus monitoring in such a host should be critical to the control of cross-species infection. In addition, previous studies showed that clade 2.3.4.4h H5N6 AIVs could infect mammals without adaptation. However, the pathogenicity and transmissibility of goose-origin clade 2.3.4.4h H5N6 AIVs in mammals remain unknown. In this study, two H5N6 AIVs were isolated from a domestic chicken (A/chicken/Hebei CK05/2019 (H5N6)) and a goose (A/goose/Hebei/GD07/2019(H5N6)). This study is the first to evaluate the pathogenicity and transmissibility of goose-origin clade 2.3.4.4h H5N6 AIVs in mammals by comparison with chicken-origin 2.3.4.4h H5N6 AIVs. The CK05 virus had an affinity for α-2,3-receptors, while the GD07 virus had an affinity for both α-2,3-and α-2,6-receptors. The GD07 virus had a higher replication capacity in vitro and more severe pathogenicity in mice than the CK05 virus. The CK05 virus could not be transmitted effectively among guinea pigs, whereas the GD07 virus could be transmitted through direct contact among guinea pigs. The results of this study indicated the potential health threat of clade 2.3.4.4h H5N6 AIVs to mammals and emphasized the importance of continuous monitoring of H5N6 AIVs, especially in waterfowl.
Keywords: H5N6; chicken; goose; pathogenicity; transmissibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pantin-Jackwood M.J., Costa-Hurtado M., Bertran K., DeJesus E., Smith D., Swayne D.E. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet. Res. 2017;48:33. doi: 10.1186/s13567-017-0435-4. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

