Genetic Diversity and Possible Origins of the Hepatitis B Virus in Siberian Natives
- PMID: 36366563
- PMCID: PMC9693834
- DOI: 10.3390/v14112465
Genetic Diversity and Possible Origins of the Hepatitis B Virus in Siberian Natives
Abstract
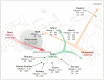
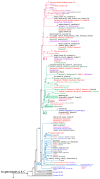
A total of 381 hepatitis B virus (HBV) DNA sequences collected from nine groups of Siberian native populations were phylogenetically analyzed along with 179 HBV strains sampled in different urban populations of former western USSR republics and 50 strains from Central Asian republics and Mongolia. Different HBV subgenotypes predominated in various native Siberian populations. Subgenotype D1 was dominant in Altaian Kazakhs (100%), Tuvans (100%), and Teleuts (100%) of southern Siberia as well as in Dolgans and Nganasans (69%), who inhabit the polar Taimyr Peninsula. D2 was the most prevalent subgenotype in the combined group of Nenets, Komi, and Khants of the northern Yamalo-Nenets Autonomous Region (71%) and in Yakuts (36%) from northeastern Siberia. D3 was the main subgenotype in South Altaians (76%) and Buryats (40%) of southeastern Siberia, and in Chukchi (51%) of the Russian Far East. Subgenotype C2 was found in Taimyr (19%) and Chukchi (27%), while subgenotype A2 was common in Yakuts (33%). In contrast, D2 was dominant (56%) in urban populations of the former western USSR, and D1 (62%) in Central Asian republics and Mongolia. Statistical analysis demonstrated that the studied groups are epidemiologically isolated from each other and might have contracted HBV from different sources during the settlement of Siberia.
Keywords: HBsAg subtypes; Siberia; Siberian natives; aboriginal population; genotypes; hepatitis B virus; molecular epidemiology; subgenotypes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bancroft W.H., Mundon F.K., Russell P.K. Detection of additional antigenic determinants of hepatitis B antigen. J. Immunol. 1972;109:842–848. - PubMed
-
- Okamoto H., Imai M., Tsuda F., Tanaka T., Miyakawa Y., Mayumi M. Point mutation in the S gene of hepatitis B virus for d/y or w/r subtypic change in two blood donors carrying a surface antigen of compound subtype adyr or adwr. J. Virol. 1987;61:3030–3034. doi: 10.1128/jvi.61.10.3030-3034.1987. - DOI - PMC - PubMed
-
- Norder H., Hammas B., Losfdahl S., Courouce A.M., Magnius L.O. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J. Gen. Virol. 1992;73:1201–1208. doi: 10.1099/0022-1317-73-5-1201. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

