Nucleus accumbens dopamine tracks aversive stimulus duration and prediction but not value or prediction error
- PMID: 36366962
- PMCID: PMC9651945
- DOI: 10.7554/eLife.82711
Nucleus accumbens dopamine tracks aversive stimulus duration and prediction but not value or prediction error
Abstract
There is active debate on the role of dopamine in processing aversive stimuli, where inferred roles range from no involvement at all, to signaling an aversive prediction error (APE). Here, we systematically investigate dopamine release in the nucleus accumbens core (NAC), which is closely linked to reward prediction errors, in rats exposed to white noise (WN, a versatile, underutilized, aversive stimulus) and its predictive cues. Both induced a negative dopamine ramp, followed by slow signal recovery upon stimulus cessation. In contrast to reward conditioning, this dopamine signal was unaffected by WN value, context valence, or probabilistic contingencies, and the WN dopamine response shifted only partially toward its predictive cue. However, unpredicted WN provoked slower post-stimulus signal recovery than predicted WN. Despite differing signal qualities, dopamine responses to simultaneous presentation of rewarding and aversive stimuli were additive. Together, our findings demonstrate that instead of an APE, NAC dopamine primarily tracks prediction and duration of aversive events.
Keywords: aversive stimuli; behavior; dopamine; motivation; neuroscience; nucleus accumbens; prediction error; rat.
© 2022, Goedhoop et al.
Conflict of interest statement
JG, Bv, RR, FV, LF, Wv, TA, IW No competing interests declared
Figures

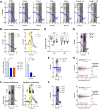
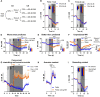
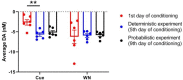
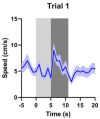
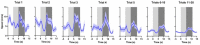
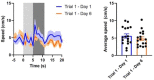
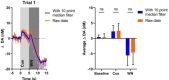
Similar articles
-
Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues.J Neurochem. 2016 Mar;136(5):1026-36. doi: 10.1111/jnc.13494. Epub 2016 Jan 24. J Neurochem. 2016. PMID: 26715366 Free PMC article.
-
Anticipation of Appetitive Operant Action Induces Sustained Dopamine Release in the Nucleus Accumbens.J Neurosci. 2023 May 24;43(21):3922-3932. doi: 10.1523/JNEUROSCI.1527-22.2023. Epub 2023 Apr 25. J Neurosci. 2023. PMID: 37185100 Free PMC article.
-
Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation.J Neurosci. 2015 Aug 19;35(33):11572-82. doi: 10.1523/JNEUROSCI.2344-15.2015. J Neurosci. 2015. PMID: 26290234 Free PMC article.
-
The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking.Brain Res Brain Res Rev. 1999 Dec;31(1):6-41. doi: 10.1016/s0165-0173(99)00023-5. Brain Res Brain Res Rev. 1999. PMID: 10611493 Review.
-
The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition?Neurosci Biobehav Rev. 2003 Oct;27(6):527-41. doi: 10.1016/j.neubiorev.2003.09.001. Neurosci Biobehav Rev. 2003. PMID: 14599434 Review.
Cited by
-
Individual differences in decision-making shape how mesolimbic dopamine regulates choice confidence and change-of-mind.bioRxiv [Preprint]. 2024 Sep 16:2024.09.16.613237. doi: 10.1101/2024.09.16.613237. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Jul 30. doi: 10.1038/s41593-025-02015-z. PMID: 39345599 Free PMC article. Updated. Preprint.
-
Striatal dopamine represents valence on dynamic regional scales.J Neurosci. 2025 Mar 17;45(17):e1551242025. doi: 10.1523/JNEUROSCI.1551-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40097183
-
Aversion-induced dopamine reductions predict drug-taking and escape behaviors.Neuropsychopharmacology. 2025 Aug;50(9):1376-1384. doi: 10.1038/s41386-025-02101-7. Epub 2025 Apr 9. Neuropsychopharmacology. 2025. PMID: 40205012
-
Addressing Altered Anticipation as a Transdiagnostic Target Through Computational Psychiatry.Biol Psychiatry Cogn Neurosci Neuroimaging. 2025 Mar 7:S2451-9022(25)00071-0. doi: 10.1016/j.bpsc.2025.02.014. Online ahead of print. Biol Psychiatry Cogn Neurosci Neuroimaging. 2025. PMID: 40058458 Review.
-
Multiple factors contribute to flight behaviors during fear conditioning.Sci Rep. 2023 Jun 27;13(1):10402. doi: 10.1038/s41598-023-37612-0. Sci Rep. 2023. PMID: 37369752 Free PMC article.
References
-
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. The Journal of Neuroscience. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

