Alveolar epithelial cells and microenvironmental stiffness synergistically drive fibroblast activation in three-dimensional hydrogel lung models
- PMID: 36366982
- PMCID: PMC9729409
- DOI: 10.1039/d2bm00827k
Alveolar epithelial cells and microenvironmental stiffness synergistically drive fibroblast activation in three-dimensional hydrogel lung models
Abstract
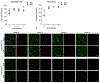
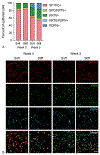
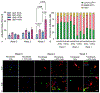
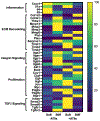
Idiopathic pulmonary fibrosis (IPF) is a devastating lung disease that progressively and irreversibly alters the lung parenchyma, eventually leading to respiratory failure. The study of this disease has been historically challenging due to the myriad of complex processes that contribute to fibrogenesis and the inherent difficulty in accurately recreating the human pulmonary environment in vitro. Here, we describe a poly(ethylene glycol) PEG hydrogel-based three-dimensional model for the co-culture of primary murine pulmonary fibroblasts and alveolar epithelial cells that reproduces the micro-architecture, cell placement, and mechanical properties of healthy and fibrotic lung tissue. Co-cultured cells retained normal levels of viability up to at least three weeks and displayed differentiation patterns observed in vivo during IPF progression. Interrogation of protein and gene expression within this model showed that myofibroblast activation required both extracellular mechanical cues and the presence of alveolar epithelial cells. Differences in gene expression indicated that cellular co-culture induced TGF-β signaling and proliferative gene expression, while microenvironmental stiffness upregulated the expression of genes related to cell-ECM interactions. This biomaterial-based cell culture system serves as a significant step forward in the accurate recapitulation of human lung tissue in vitro and highlights the need to incorporate multiple factors that work together synergistically in vivo into models of lung biology of health and disease.
Conflict of interest statement
Conflicts of Interest
Chelsea Magin reports a relationship with Vanderbilt University that includes: speaking and lecture fees. Chelsea Magin has patent #PCT/US2019/012722 pending to University of Colorado.
Figures


References
-
- L R, HR C and MG J, Lancet (London, England), 2017, 389. - PubMed
-
- Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, Takeuchi M, Raghu G, Kudoh S, Nukiwa T and the J Pirfenidone Clinical Study Group in, European Respiratory Journal, 2010, 35, 821. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B and Collard HR, New England Journal of Medicine, 2014, 370, 2071–2082. - PubMed
-
- Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH, Tsidiridis G, Lange M, Mattner LF, Yee M, Ogar P, Sengupta A, Kukhtevich I, Schneider R, Zhao Z, Voss C, Stoeger T, Neumann JHL, Hilgendorff A, Behr J, O’Reilly M, Lehmann M, Burgstaller G, Königshoff M, Chapman HA, Theis FJ and Schiller HB, Nature Communications, 2020, 11, 1–20. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

