Magnetic resonance radiomic feature performance in pulmonary nodule classification and impact of segmentation variability on radiomics
- PMID: 36367095
- PMCID: PMC9733623
- DOI: 10.1259/bjr.20220230
Magnetic resonance radiomic feature performance in pulmonary nodule classification and impact of segmentation variability on radiomics
Abstract
Objective: Investigate the performance of multiparametric MRI radiomic features, alone or combined with current standard-of-care methods, for pulmonary nodule classification. Assess the impact of segmentation variability on feature reproducibility and reliability.
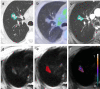
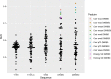
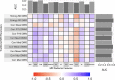
Methods: Radiomic features were extracted from 74 pulmonary nodules of 68 patients who underwent nodule resection or biopsy after MRI exam. The MRI features were compared with histopathology and conventional quantitative imaging values (maximum standardized uptake value [SUVmax] and mean Hounsfield unit [HU]) to determine whether MRI radiomic features can differentiate types of nodules and associate with SUVmax and HU using Wilcoxon rank sum test and linear regression. Diagnostic performance of features and four machine learning (ML) models were evaluated with area under the receiver operating characteristic curve (AUC) and 95% confidence intervals (CIs). Concordance correlation coefficient (CCC) assessed the segmentation variation impact on feature reproducibility and reliability.
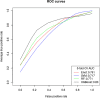
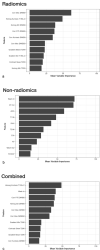
Results: Elevn diffusion-weighted features distinguished malignant from benign nodules (adjusted p < 0.05, AUC: 0.73-0.81). No features differentiated cancer types. Sixty-seven multiparametric features associated with mean CT HU and 14 correlated with SUVmax. All significant MRI features outperformed traditional imaging parameters (SUVmax, mean HU, apparent diffusion coefficient [ADC], T1, T2, dynamic contrast-enhanced imaging values) in distinguishing malignant from benign nodules with some achieving statistical significance (p < 0.05). Adding ADC and smoking history improved feature performance. Machine learning models demonstrated strong performance in nodule classification, with extreme gradient boosting (XGBoost) having the highest discrimination (AUC = 0.83, CI=[0.727, 0.932]). We found good to excellent inter- and intrareader feature reproducibility and reliability (CCC≥0.80).
Conclusion: Eleven MRI radiomic features differentiated malignant from benign lung nodules, outperforming traditional quantitative methods. MRI radiomic ML models demonstrated good nodule classification performances with XGBoost superior to three others. There was good to excellent inter- and intrareader feature reproducibility and reliability.
Advances in knowledge: Our study identified MRI radiomic features that successfully differentiated malignant from benign lung nodules and demonstrated high performance of our MR radiomic feature-based ML models for nodule classification. These new findings could help further establish thoracic MRI as a non-invasive and radiation-free alternative to standard practice for pulmonary nodule assessment.
Figures


References
-
- Common Cancer Types NIH . National Cancer Institutie . Available from : https://www.cancer.gov/types/common-cancers
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

