Rapid parallel generation of a fluorescently barcoded drop library from a microtiter plate using the plate-interfacing parallel encapsulation (PIPE) chip
- PMID: 36367139
- PMCID: PMC10016142
- DOI: 10.1039/d2lc00909a
Rapid parallel generation of a fluorescently barcoded drop library from a microtiter plate using the plate-interfacing parallel encapsulation (PIPE) chip
Abstract
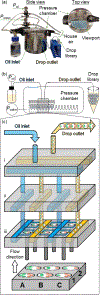
In drop-based microfluidics, an aqueous sample is partitioned into drops using individual pump sources that drive water and oil into a drop-making device. Parallelization of drop-making devices is necessary to achieve high-throughput screening of multiple experimental conditions, especially in time-sensitive studies. Here, we present the plate-interfacing parallel encapsulation (PIPE) chip, a microfluidic chip designed to generate 50 to 90 μm diameter drops of up to 96 different conditions in parallel by interfacing individual drop makers with a standard 384-well microtiter plate. The PIPE chip is used to generate two types of optically barcoded drop libraries consisting of two-color fluorescent particle combinations: a library of 24 microbead barcodes and a library of 192 quantum dot barcodes. Barcoded combinations in the drop libraries are rapidly measured within a microfluidic device using fluorescence detection and distinct barcoded populations in the fluorescence drop data are identified using DBSCAN data clustering. Signal analysis reveals that particle size defines the source of dominant noise present in the fluorescence intensity distributions of the barcoded drop populations, arising from Poisson loading for microbeads and shot noise for quantum dots. A barcoded population from a drop library is isolated using fluorescence-activated drop sorting, enabling downstream analysis of drop contents. The PIPE chip can improve multiplexed high-throughput assays by enabling simultaneous encapsulation of barcoded samples stored in a microtiter plate and reducing sample preparation time.
Conflict of interest statement
Conflicts of Interest
There are no conflicts to declare.
Figures




Similar articles
-
High throughput single-cell and multiple-cell micro-encapsulation.J Vis Exp. 2012 Jun 15;(64):e4096. doi: 10.3791/4096. J Vis Exp. 2012. PMID: 22733254 Free PMC article.
-
High-throughput fluorescence detection using an integrated zone-plate array.Lab Chip. 2010 Apr 7;10(7):852-6. doi: 10.1039/b923554j. Epub 2010 Jan 18. Lab Chip. 2010. PMID: 20300671
-
A microfluidic device with microbead array for sensitive virus detection and genotyping using quantum dots as fluorescence labels.Biosens Bioelectron. 2010 Jul 15;25(11):2402-7. doi: 10.1016/j.bios.2010.02.032. Epub 2010 Mar 6. Biosens Bioelectron. 2010. PMID: 20483585
-
Industrial lab-on-a-chip: design, applications and scale-up for drug discovery and delivery.Adv Drug Deliv Rev. 2013 Nov;65(11-12):1626-63. doi: 10.1016/j.addr.2013.07.017. Epub 2013 Jul 27. Adv Drug Deliv Rev. 2013. PMID: 23899864 Review.
-
Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review.Biosens Bioelectron. 2019 Feb 1;126:697-706. doi: 10.1016/j.bios.2018.11.032. Epub 2018 Nov 20. Biosens Bioelectron. 2019. PMID: 30544083 Review.
Cited by
-
Open-source pneumatic pressure pump for drop-based microfluidic flow controls.Eng Res Express. 2023 Sep;5(3):035014. doi: 10.1088/2631-8695/ace299. Epub 2023 Jul 12. Eng Res Express. 2023. PMID: 39649444 Free PMC article.
References
-
- Guo MT, Rotem A, Heyman JA and Weitz DA, Lab on a Chip, 2012, 12, 2146–2155. - PubMed

