HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review)
- PMID: 36367182
- PMCID: PMC9685368
- DOI: 10.3892/or.2022.8443
HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review)
Abstract
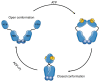
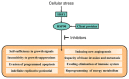
Heat shock protein 90 (HSP90) is a vital chaperone protein, regulating signaling pathways and correcting misfolded proteins in cancer cells by interacting with oncogenic client proteins and co‑chaperones. The inhibition of HSP90 chaperone machinery has been demonstrated as a potential approach with which to inhibit tumor survival, proliferation, invasion and migration. Numerous HSP90 inhibitors have been reported and have exhibited value as cancer‑targeted therapies by interrupting the ATPase activity of HSP90, thus suppressing the oncogenic pathways in cancer cells. These inhibitors have been classified into three categories: i) N‑terminal domain (NTD) inhibitors; ii) C‑terminal domain (CTD) inhibitors; and iii) isoform‑selective inhibitors. However, none of these HSP90 inhibitors are used as clinical treatments. The major limiting factors can be summarized into drug resistance, dose‑limiting toxicity and poor pharmacokinetic profiles. Novel HSP90‑targeted compounds are constantly being discovered and tested for their antitumor efficacy in preclinical and clinical trials, highlighting the prospect of the use of HSP90 inhibitors as cancer‑targeted therapies. Additionally, improved antitumor effects have been observed when HSP90 inhibitors are used in combination with chemotherapy, targeted agents, or immunotherapy. In the present review, the effects of HSP90 inhibitors on the management of the cancer process are discussed and previous and novel HSP90‑based therapeutic strategies in cancer treatment are summarized. Furthermore, prospective HSP90‑targeting candidates are proposed for their future evaluation as cancer treatments.
Keywords: HSP90 inhibitors; combination therapies; heat shock protein 90; selective inhibitors; targeted therapies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

