Rescuing Cardiac Cells and Improving Cardiac Function by Targeted Delivery of Oxygen-Releasing Nanoparticles after or Even before Acute Myocardial Infarction
- PMID: 36367231
- PMCID: PMC9930176
- DOI: 10.1021/acsnano.2c10043
Rescuing Cardiac Cells and Improving Cardiac Function by Targeted Delivery of Oxygen-Releasing Nanoparticles after or Even before Acute Myocardial Infarction
Abstract
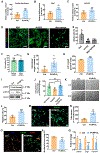
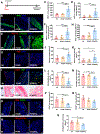
Myocardial infarction (MI) causes massive cell death due to restricted blood flow and oxygen deficiency. Rapid and sustained oxygen delivery following MI rescues cardiac cells and restores cardiac function. However, current oxygen-generating materials cannot be administered during acute MI stage without direct injection or suturing methods, both of which risk rupturing weakened heart tissue. Here, we present infarcted heart-targeting, oxygen-releasing nanoparticles capable of being delivered by intravenous injection at acute MI stage, and specifically accumulating in the infarcted heart. The nanoparticles can also be delivered before MI, then gather at the injured area after MI. We demonstrate that the nanoparticles, delivered either pre-MI or post-MI, enhance cardiac cell survival, stimulate angiogenesis, and suppress fibrosis without inducing substantial inflammation and reactive oxygen species overproduction. Our findings demonstrate that oxygen-delivering nanoparticles can provide a nonpharmacological solution to rescue the infarcted heart during acute MI and preserve heart function.
Keywords: acute myocardial infarction; controlled release of oxygen; myocardial repair; nanoparticles; targeted delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


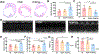
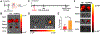

References
-
- Fryar CD; Chen T-C; Li X Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Br. 2012, 103, 1–8. - PubMed
-
- Shuvy M; Atar D; Gabriel Steg P; Halvorsen S; Jolly S; Yusuf S; Lotan C Oxygen therapy in acute coronary syndrome: are the benefits worth the risk? Eur. Heart J 2013, 34 (22), 1630–1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

