Co‑cultivation of anaerobic fungi with Clostridium acetobutylicum bolsters butyrate and butanol production from cellulose and lignocellulose
- PMID: 36367297
- PMCID: PMC9923384
- DOI: 10.1093/jimb/kuac024
Co‑cultivation of anaerobic fungi with Clostridium acetobutylicum bolsters butyrate and butanol production from cellulose and lignocellulose
Abstract
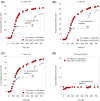
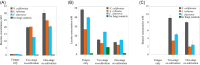
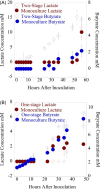
A system for co-cultivation of anaerobic fungi with anaerobic bacteria was established based on lactate cross-feeding to produce butyrate and butanol from plant biomass. Several co-culture formulations were assembled that consisted of anaerobic fungi (Anaeromyces robustus, Neocallimastix californiae, or Caecomyces churrovis) with the bacterium Clostridium acetobutylicum. Co-cultures were grown simultaneously (e.g., 'one pot'), and compared to cultures where bacteria were cultured in fungal hydrolysate sequentially. Fungal hydrolysis of lignocellulose resulted in 7-11 mM amounts of glucose and xylose, as well as acetate, formate, ethanol, and lactate to support clostridial growth. Under these conditions, one-stage simultaneous co-culture of anaerobic fungi with C. acetobutylicum promoted the production of butyrate up to 30 mM. Alternatively, two-stage growth slightly promoted solventogenesis and elevated butanol levels (∼4-9 mM). Transcriptional regulation in the two-stage growth condition indicated that this cultivation method may decrease the time required to reach solventogenesis and induce the expression of cellulose-degrading genes in C. acetobutylicum due to relieved carbon-catabolite repression. Overall, this study demonstrates a proof of concept for biobutanol and bio-butyrate production from lignocellulose using an anaerobic fungal-bacterial co-culture system.
Keywords: Anaerobic fungi; Biofuel; Clostridia; Consortia; RNA-Seq.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Recent advances in n-butanol and butyrate production using engineered Clostridium tyrobutyricum.World J Microbiol Biotechnol. 2020 Aug 14;36(9):138. doi: 10.1007/s11274-020-02914-2. World J Microbiol Biotechnol. 2020. PMID: 32794091 Review.
-
Clostridium strain selection for co-culture with Bacillus subtilis for butanol production from agave hydrolysates.Bioresour Technol. 2019 Mar;275:410-415. doi: 10.1016/j.biortech.2018.12.085. Epub 2018 Dec 27. Bioresour Technol. 2019. PMID: 30605828
-
Improvement of butanol production in Clostridium acetobutylicum through enhancement of NAD(P)H availability.J Ind Microbiol Biotechnol. 2018 Nov;45(11):993-1002. doi: 10.1007/s10295-018-2068-7. Epub 2018 Aug 23. J Ind Microbiol Biotechnol. 2018. PMID: 30141107
-
New insights into the butyric acid metabolism of Clostridium acetobutylicum.Appl Microbiol Biotechnol. 2012 Dec;96(5):1325-39. doi: 10.1007/s00253-012-4109-x. Epub 2012 May 12. Appl Microbiol Biotechnol. 2012. PMID: 22576943
-
Transcriptomic studies of solventogenic clostridia, Clostridium acetobutylicum and Clostridium beijerinckii.Biotechnol Adv. 2022 Sep;58:107889. doi: 10.1016/j.biotechadv.2021.107889. Epub 2021 Dec 17. Biotechnol Adv. 2022. PMID: 34929313 Review.
Cited by
-
From pre-culture to solvent: current trends in Clostridium acetobutylicum cultivation.Appl Microbiol Biotechnol. 2025 Feb 18;109(1):47. doi: 10.1007/s00253-025-13428-y. Appl Microbiol Biotechnol. 2025. PMID: 39964448 Free PMC article. Review.
-
Modeling control and transduction of electrochemical gradients in acid-stressed bacteria.iScience. 2023 Jun 17;26(7):107140. doi: 10.1016/j.isci.2023.107140. eCollection 2023 Jul 21. iScience. 2023. PMID: 37404371 Free PMC article.
-
Patterns and determinants of the global herbivorous mycobiome.Nat Commun. 2023 Jun 26;14(1):3798. doi: 10.1038/s41467-023-39508-z. Nat Commun. 2023. PMID: 37365172 Free PMC article.
-
Fungal Coculture: Unlocking the Potential for Efficient Bioconversion of Lignocellulosic Biomass.J Fungi (Basel). 2025 Jun 17;11(6):458. doi: 10.3390/jof11060458. J Fungi (Basel). 2025. PMID: 40558970 Free PMC article. Review.
-
Multiomics of yaks reveals significant contribution of microbiome into host metabolism.NPJ Biofilms Microbiomes. 2024 Nov 21;10(1):133. doi: 10.1038/s41522-024-00609-2. NPJ Biofilms Microbiomes. 2024. PMID: 39572587 Free PMC article.
References
-
- Al-Shorgani N. K. N., Tibin E. M., Ali E., Hamid A. A., Yusoff W. M. W., Kalil M. S. (2014). Biohydrogen production from agroindustrial wastes via Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). Clean Technologies and Environmental Policy, 16(1), 11–21. 10.1007/s10098-013-0586-6 - DOI

