Engineering and Validation of a Peptide-Stabilized Poly(lactic- co-glycolic) Acid Nanoparticle for Targeted Delivery of a Vascular Disruptive Agent in Cancer Therapy
- PMID: 36367382
- PMCID: PMC10292766
- DOI: 10.1021/acs.bioconjchem.2c00418
Engineering and Validation of a Peptide-Stabilized Poly(lactic- co-glycolic) Acid Nanoparticle for Targeted Delivery of a Vascular Disruptive Agent in Cancer Therapy
Abstract
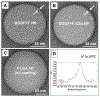
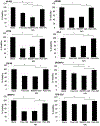
Developing a biocompatible and biodegradable nanoparticle (NP) carrier that integrates drug-loading capability, active targeting, and imaging modality is extremely challenging. Herein, we report an NP with a core of poly(lactic-co-glycolic) acid (PLGA) chemically modified with the drug combretastatin A4 (CA4), a vascular disrupting agent (VDA) in clinical development for ovarian cancer (OvCA) therapy. The NP is stabilized with a short arginine-glycine-aspartic acid-phenylalanine x3 (RGDFFF) peptide via self-assembly of the peptide on the PLGA surface. Importantly, the use of our RGDFFF coating replaces the commonly used polyethylene glycol (PEG) polymer that itself often induces an unwanted immunogenic response. In addition, the RGD motif of the peptide is well-known to preferentially bind to αvβ3 integrin that is implicated in tumor angiogenesis and is exploited as the NP's targeting component. The NP is enhanced with an optical imaging fluorophore label via chemical modification of the PLGA. The RGDFFF-CA4 NPs are synthesized using a nanoprecipitation method and are ∼75 ± 3.7 nm in diameter, where a peptide coating comprises a 2-3 nm outer layer. The NPs are serum stable for 72 h. In vitro studies using human umbilical cord vascular endothelial cells (HUVEC) confirmed the high uptake and biological activity of the RGDFFF-CA4 NP. NP uptake and viability reduction were demonstrated in OvCA cells grown in culture, and the NPs efficiently accumulated in tumors in a preclinical OvCA mouse model. The RGDFFF NP did not induce an inflammatory response when cultured with immune cells. Finally, the NP was efficiently taken up by patient-derived OvCA cells, suggesting a potential for future clinical applications.
Figures







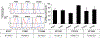
References
-
- Peer D; Karp JM; Hong S; Farokhzad OC; Margalit R; Langer R, Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007, 2 (12), 751–60. - PubMed
-
- Jafari S; Derakhshankhah H; Alaei L; Fattahi A; Varnamkhasti BS; Saboury AA, Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed Pharmacother 2019, 109, 1100–1111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

