Mosquito aquatic habitat modification and manipulation interventions to control malaria
- PMID: 36367444
- PMCID: PMC9651131
- DOI: 10.1002/14651858.CD008923.pub3
Mosquito aquatic habitat modification and manipulation interventions to control malaria
Abstract
Background: Larval source management (LSM) may help reduce Plasmodium parasite transmission in malaria-endemic areas. LSM approaches include habitat modification (permanently or temporarily reducing mosquito breeding aquatic habitats); habitat manipulation (temporary or recurrent change to environment); or use of chemical (e.g. larviciding) or biological agents (e.g. natural predators) to breeding sites. We examined the effectiveness of habitat modification or manipulation (or both), with and without larviciding. This is an update of a review published in 2013.
Objectives: 1. To describe and summarize the interventions on mosquito aquatic habitat modification or mosquito aquatic habitat manipulation, or both, on malaria control. 2. To evaluate the beneficial and harmful effects of mosquito aquatic habitat modification or mosquito aquatic habitat manipulation, or both, on malaria control.
Search methods: We used standard, extensive Cochrane search methods. The latest search was from January 2012 to 30 November 2021.
Selection criteria: Randomized controlled trials (RCT) and non-randomized intervention studies comparing mosquito aquatic habitat modification or manipulation (or both) to no treatment or another active intervention. We also included uncontrolled before-after (BA) studies, but only described and summarized the interventions from studies with these designs. Primary outcomes were clinical malaria incidence, malaria parasite prevalence, and malaria parasitaemia incidence.
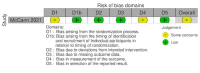
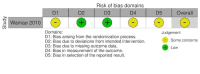
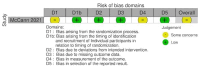
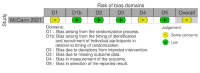
Data collection and analysis: We used standard Cochrane methods. We assessed risk of bias using the Cochrane RoB 2 tool for RCTs and the ROBINS-I tool for non-randomized intervention studies. We used a narrative synthesis approach to systematically describe and summarize all the interventions included within the review, categorized by the type of intervention (habitat modification, habitat manipulation, combination of habitat modification and manipulation). Our primary outcomes were 1. clinical malaria incidence; 2. malaria parasite prevalence; and 3. malaria parasitaemia incidence. Our secondary outcomes were 1. incidence of severe malaria; 2. anaemia prevalence; 3. mean haemoglobin levels; 4. mortality rate due to malaria; 5. hospital admissions for malaria; 6. density of immature mosquitoes; 7. density of adult mosquitoes; 8. sporozoite rate; 9. entomological inoculation rate; and 10.
Harms: We used the GRADE approach to assess the certainty of the evidence for each type of intervention.
Main results: Sixteen studies met the inclusion criteria. Six used an RCT design, six used a controlled before-after (CBA) study design, three used a non-randomized controlled design, and one used an uncontrolled BA study design. Eleven studies were conducted in Africa and five in Asia. Five studies reported epidemiological outcomes and 15 studies reported entomological outcomes. None of the included studies reported on the environmental impacts associated with the intervention. For risk of bias, all trials had some concerns and other designs ranging from moderate to critical. Ten studies assessed habitat manipulation (temporary change to the environment). This included water management (spillways across streams; floodgates; intermittent flooding; different drawdown rates of water; different flooding and draining regimens), shading management (shading of drainage channels with different plants), other/combined management approaches (minimal tillage; disturbance of aquatic habitats with grass clearing and water replenishment), which showed mixed results for entomological outcomes. Spillways across streams, faster drawdown rates of water, shading drainage canals with Napier grass, and using minimal tillage may reduce the density of immature mosquitoes (range of effects from 95% reduction to 1.7 times increase; low-certainty evidence), and spillways across streams may reduce densities of adult mosquitoes compared to no intervention (low-certainty evidence). However, the effect of habitat manipulation on malaria parasite prevalence and clinical malaria incidence is uncertain (very low-certainty evidence). Two studies assessed habitat manipulation with larviciding. This included reducing or removal of habitat sites; and drain cleaning, grass cutting, and minor repairs. It is uncertain whether drain cleaning, grass cutting, and minor repairs reduces malaria parasite prevalence compared to no intervention (odds ratio 0.59, 95% confidence interval (CI) 0.42 to 0.83; very low-certainty evidence). Two studies assessed combination of habitat manipulation and permanent change (habitat modification). This included drainage canals, filling, and planting of papyrus and other reeds for shading near dams; and drainage of canals, removal of debris, land levelling, and filling ditches. Studies did not report on epidemiological outcomes, but entomological outcomes suggest that such activities may reduce the density of adult mosquitoes compared to no intervention (relative risk reduction 0.49, 95% CI 0.47 to 0.50; low-certainty evidence), and preventing water stagnating using drainage of canals, removal of debris, land levelling, and filling ditches may reduce the density of immature mosquitoes compared to no intervention (ranged from 10% to 55% reductions; low-certainty evidence). Three studies assessed combining manipulation and modification with larviciding. This included filling or drainage of water bodies; filling, draining, or elimination of rain pools and puddles at water supply points and stream bed pools; and shoreline work, improvement and maintenance to drainage, clearing vegetation and undergrowth, and filling pools. There were mixed effect sizes for the reduction of entomological outcomes (moderate-certainty evidence). However, filling or draining water bodies probably makes little or no difference to malaria parasite prevalence, haemoglobin levels, or entomological inoculation rate when delivered with larviciding compared to no intervention (moderate-certainty evidence).
Authors' conclusions: Habitat modification and manipulation interventions for preventing malaria has some indication of benefit in both epidemiological and entomological outcomes. While the data are quite mixed and further studies could help improve the knowledge base, these varied approaches may be useful in some circumstances.
Copyright © 2022 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
EM: none.
GY: none.
RR is a Vice President of Global Health for RTI International, a non‐profit research institute based in North Carolina, USA.
JLB: consultancy fees from undertaking independent statistical review for Danone Nutricia Research, and from providing statistical expertise to the Food Standards Agency, which are both outside the subject of this review. JLB is a Content Editor for the Cochrane Diagnostic Accuracy Reviews Editorial Team.
Figures













Update of
-
Mosquito larval source management for controlling malaria.Cochrane Database Syst Rev. 2013 Aug 29;2013(8):CD008923. doi: 10.1002/14651858.CD008923.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2022 Nov 11;11:CD008923. doi: 10.1002/14651858.CD008923.pub3. PMID: 23986463 Free PMC article. Updated.
References
References to studies included in this review
Castro 2009 {published data only}
Djegbe 2020 {published data only}
-
- Djegbe I, Zinsou M, Flavien Dovonou E, Tchigossou G, Soglo M, Adeoti R, et al. Minimal tillage and intermittent flooding farming systems show a potential reduction in the proliferation of Anopheles mosquito larvae in a rice field in Malanville, Northern Benin. Malaria Journal 2020;19:333. - PMC - PubMed
Imbahale 2011 {published data only}
Imbahale 2012 {published data only}
Kibret 2018 {published data only}
Lee 2010 {published data only}
McCann 2021 {published data only}
-
- McCann RS, Kabaghe AN, Moraga P, Gowelo S, Mburu MM, Tizifa T, et al. The effect of community-driven larval source management and house improvement on malaria transmission when added to the standard malaria control strategies in Malawi: a cluster-randomized controlled trial. Malaria Journal 2021;20:232. - PMC - PubMed
Munga 2013 {published data only}
Mutero 2000 {published data only}
-
- Mutero CM, Blank H, Konradsen F, Hoek W. Water management for controlling the breeding of Anopheles mosquitoes in rice irrigation schemes in Kenya. Acta Tropica 2000;76(3):253-63. - PubMed
Sahu 2014 {published data only}
Samnotra 1980 {published data only}
-
- Samnotra K, Kumar P. Field evaluation of pirimiphos-methyl as a mosquito larvicide in an urban area of India as part of the national malaria eradication programme. Mosquito News 1980;40:257-63.
Santiago 1960 {published data only}
-
- Santiago D. Malaria control by automatic flushing of streams. Philippine Journal Science 1960;8:373-95.
Sharma 2008 {published data only}
-
- Sharma SK, Tyagi PK, Upadhyay AK, Haque MA, Adak T, Dash AP. Building small dams can decrease malaria: a comparative study from Sundargarh District, Orissa, India. Acta Tropica 2008;107(2):174-8. - PubMed
Shililu 2007 {published data only}
-
- Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. American Journal of Tropical Medicine and Hygiene 2007;76(1):103-10. - PubMed
Wamae 2010 {published data only}
Yohannes 2005 {published data only}
-
- Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A, Byass P, et al. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Tropical Medicine and International Health 2005;10(12):1274-85. - PubMed
References to studies excluded from this review
Amerasinghe 1991 {published data only}
-
- Amerasinghe FP, Amerasinghe PH, Peiris JS, Wirtz RA. Anopheline ecology and malaria infection during the irrigation development of an area of the Mahaweli Project, Sri Lanka. American Journal of Tropical Medicine and Hygiene 1991;45(2):226-35. - PubMed
Balfour 1936 {published data only}
-
- Balfour MC. Some features of malaria in Greece and experience with its control. Rivista di Malariologia 1936;15:114-31.
Clark 2012 {published data only}
-
- Clark GG, Rubio-Palis Y. Mosquito vector biology and control in Latin America 22nd symposium. Journal of the American Mosquito Control Association 2012;28(2):102-10. - PubMed
Clark 2013 {published data only}
-
- Clark GG, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 23rd symposium. Journal of the American Mosquito Control Association 2013;29(3):251-69. - PubMed
Clark 2014 {published data only}
-
- Clark GG, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 24th symposium. Journal of the American Mosquito Control Association 2014;30(3):204-14. - PubMed
Cohnstaedt 2016 {published data only}
-
- Cohnstaedt LW, Alfonso-Para C, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 26th Symposium. Journal of American Mosquito Control Association 2016;32(4):315-22. - PubMed
Cohnstaedt 2017 {published data only}
-
- Cohnstaedt LW, Alfonso-Parra C, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 27th symposium. Journal of the American Mosquito Control Association 2017;33(3):215-24. - PubMed
Frake 2017 {published data only}
-
- Frake A, Messina J, Walker ED, Zulu L, Mangani C, Mkwaila W, et al. Scaling irrigation and malaria risk in Malawi. American Journal of Tropical Medicine and Hygiene 2017;97(5):509.
Getachew 2020 {published data only}
Gezie 2018 {published data only}
-
- Gezie A, Assefa WW, Getnet B, Anteneh W, Dejen E, Mereta ST. Potential impacts of water hyacinth invasion and management on water quality and human health in Lake Tana watershed, Northwest Ethiopia. Biological Invasions 2018;20(9):2517-34.
Jaleta 2013 {published data only}
Kibret 2014 {published data only}
Kibret 2019 {published data only}
Kiszewski 2014 {published data only}
-
- Kiszewski AE, Teffera Z, Wondafrash M, Ravesi M, Pollack RJ. Ecological succession and its impact on malaria vectors and their predators in borrow pits in western Ethiopia. Journal of Vector Ecology 2014;39(2):414-23. - PubMed
Laporta 2019 {published data only}
-
- Laporta GZ. Amazonian rainforest loss and declining malaria burden in Brazil. Lancet Planetary Health 2019;3(1):e4-5. - PubMed
Nasreen 2016 {published data only}
-
- Nasreen A, Nagpal BN, Kapoor N, Srivastava A, Gupta HP, Saxena R, et al. Impact of ecological and climatic changes on vectors of malaria in four North-Eastern states of India. Indian Journal of Ecology 2016;43(1):1-15.
Ohta 2014 {published data only}
-
- Ohta S, Kaga T. Effect of irrigation systems on temporal distribution of malaria vectors in semi-arid regions. International Journal of Biometeorology 2014;58(3):349-59. - PubMed
Phiri 2021 {published data only}
Saxena 2014 {published data only}
-
- Saxena R, Nagpal BN, Singh VP, Srivastava A, Dev V, Sharma MC, et al. Impact of deforestation on known malaria vectors in Sonitpur district of Assam, India. Journal of Vector Borne Diseases 2014;51(3):211-5. - PubMed
Srivastava 2013 {published data only}
-
- Srivastava AK, Kharbuli B, Shira DS, Sood A. Effect of land use and land cover modification on distribution of anopheline larval habitats in Meghalaya, India. Journal of Vector Borne Diseases 2013;50(2):121-6. - PubMed
Tchoumbou 2020 {published data only}
-
- Tchoumbou MA, Mayi MP, Malange EN, Foncha FD, Kowo C, Fru-cho J, et al. Effect of deforestation on prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. International Journal for Parasitology 2020;50(1):63-73. - PubMed
Thapar 2019 {published data only}
-
- Thapar R, Singh S, Srivastava P. The study on impact of Ujina irrigation canal on malaria transmission in District Nuh (Erstwhile Mewat), Haryana. Journal of Communicable Diseases 2019;51(3):46-54.
van den Berg 2018 {published data only}
References to ongoing studies
Additional references
Atkinson 2011
Choi 2019
Cochrane 2009
-
- Cochrane Effective Practice and Organisation of Care Group (EPOC). Risk of bias guidelines. EPOC Author Resources. epoc.cochrane.org/ (accessed 30 September 2020).
Guyatt 2008
Higgins 2003
Keiser 2005
-
- Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infectious Diseases 2005;5(11):695-708. - PubMed
McCann 2017
-
- McCann RS, den Berg H, Diggle PJ, Vugt M, Terlouw DJ, Phiri KS, et al. Assessment of the effect of larval source management and house improvement on malaria transmission when added to standard malaria control strategies in southern Malawi: study protocol for a cluster-randomised controlled trial. BMC Infectious Diseases 2017;17:639. - PMC - PubMed
Muema 2017
Schünemann 2019
Sterne 2016
Sterne 2019
-
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. - PubMed
Vontas 2014
-
- Vontas J, Moore S, Kleinschmidt I, Ranson H, Lindsay S, Lengeler C, et al. Framework for rapid assessment and adoption of new vector control tools. Trends in Parasitology 2014;30(4):191-204. - PubMed
Walshe 2017
WHO 2011
-
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity, 2011. apps.who.int/iris/handle/10665/85839 (accessed 29 September 2020).
WHO 2012
-
- World Health Organization. Handbook for integrated vector management, June 2012. www.who.int/publications/i/item/9789241502801 (accessed 19 May 2021).
WHO 2013
-
- World Health Organization. Larval source management: a supplementary measure for malaria vector control, July 2013. www.who.int/publications/i/item/9789241505604 (accessed 29 September 2020).
WHO 2015a
-
- World Health Organization. Global technical strategy for malaria 2016–2030, June 2015. www.who.int/publications/i/item/9789240031357 (accessed 19 May 2021).
WHO 2015b
-
- World Health Organization. Guidelines for the treatment of malaria. Third edition, April 2015. www.afro.who.int/publications/guidelines-treatment-malaria-third-edition (accessed 29 September 2020).
WHO 2017
-
- World Health Organization. Global vector control response 2017–2030, October 2017. www.who.int/publications/i/item/9789241512978 (accessed 19 May 2021).
WHO 2019a
-
- World Health Organization (WHO). World malaria report 2019. www.who.int/publications/i/item/9789241565721 (accessed 29 September 2020).
WHO 2019b
-
- World Health Organization (WHO). Guidelines for malaria vector control, February 2019. apps.who.int/iris/bitstream/handle/10665/310862/9789241550499-eng.pdf (accessed 29 September 2020).
WHO 2020
-
- World Health Organization. World malaria report 2020 – 20 years of global progress & challenges. www.who.int/publications/i/item/9789240015791 (accessed 19 May 2021).
References to other published versions of this review
Thwing 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

