Novel ocular immunotherapy induces tumor regression in an equine model of ocular surface squamous neoplasia
- PMID: 36367558
- PMCID: PMC10992022
- DOI: 10.1007/s00262-022-03321-2
Novel ocular immunotherapy induces tumor regression in an equine model of ocular surface squamous neoplasia
Abstract
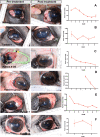
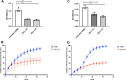
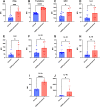
Ocular surface squamous neoplasia (OSSN) is the major cause of corneal cancer in man and horses worldwide, and the prevalence of OSSN is increasing due to greater UVB exposure globally. Currently, there are no approved treatments for OSSN in either species, and most patients are managed with surgical excision or off-label treatment with locally injected interferon alpha, or topically applied cytotoxic drugs such as mitomycin C. A more broadly effective and readily applied immunotherapy could exert a significant impact on management of OSSN worldwide. We therefore evaluated the effectiveness of a liposomal TLR complex (LTC) immunotherapy, which previously demonstrated strong antiviral activity in multiple animal models following mucosal application, for ocular antitumor activity in a horse spontaneous OSSN model. In vitro studies demonstrated strong activation of interferon responses in horse leukocytes by LTC and suppression of OSSN cell growth and migration. In a trial of 8 horses (9 eyes), treatment with topical or perilesional LTC resulted in an overall tumor response rate of 67%, including durable regression of large OSSN tumors. Repeated treatment with LTC ocular immunotherapy was also very well tolerated clinically. We conclude therefore that ocular immunotherapy with LTC warrants further investigation as a novel approach to management of OSSN in humans.
Keywords: Horse; Immunotherapy; Ocular surface squamous neoplasia.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Authors Steven Dow and Lyndah Chow declare that they are among several holders of an issued US patent related to the LTC technology. All other authors declared no potential conflicts of interest.
The authors have read the journal’s policy and confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. Owners provided consent for enrollment of client-owned patients in this clinical study. SD and LC declare that they are among several holders of an issued US patent related to the LTC technology. All other authors declared no potential conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

