Intracranial Pressure Dysfunction Following Severe Intracerebral Hemorrhage in Middle-Aged Rats
- PMID: 36367666
- PMCID: PMC10640482
- DOI: 10.1007/s12975-022-01102-8
Intracranial Pressure Dysfunction Following Severe Intracerebral Hemorrhage in Middle-Aged Rats
Abstract
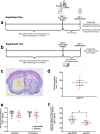
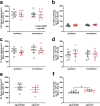
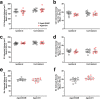
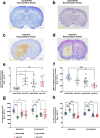
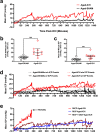
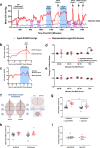
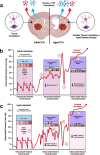
Rising intracranial pressure (ICP) aggravates secondary injury and heightens risk of death following intracerebral hemorrhage (ICH). Long-recognized compensatory mechanisms that lower ICP include reduced cerebrospinal fluid and venous blood volumes. Recently, we identified another compensatory mechanism in severe stroke, a decrease in cerebral parenchymal volume via widespread reductions in cell volume and extracellular space (tissue compliance). Here, we examined how age affects tissue compliance and ICP dynamics after severe ICH in rats (collagenase model). A planned comparison to historical young animal data revealed that aged SHAMs (no stroke) had significant cerebral atrophy (9% reduction, p ≤ 0.05), ventricular enlargement (9% increase, p ≤ 0.05), and smaller CA1 neuron volumes (21%, p ≤ 0.05). After ICH in aged animals, contralateral striatal neuron density and CA1 astrocyte density significantly increased (12% for neurons, 7% for astrocytes, p ≤ 0.05 vs. aged SHAMs). Unlike young animals, other regions in aged animals did not display significantly reduced cell soma volume despite a few trends. Nonetheless, overall contralateral hemisphere volume was 10% smaller in aged ICH animals compared to aged SHAMs (p ≤ 0.05). This age-dependent pattern of tissue compliance is not due to absent ICH-associated mass effect (83.2 mm3 avg. bleed volume) as aged ICH animals had significantly elevated mean and peak ICP (p ≤ 0.01), occurrence of ICP spiking events, as well as bilateral evidence of edema (e.g., 3% in injured brain, p ≤ 0.05 vs. aged SHAMs). Therefore, intracranial compliance reserve changes with age; after ICH, these and other age-related changes may cause greater fluctuation from baseline, increasing the chance of adverse outcomes like mortality.
Keywords: Aging; Cell volume; Edema; Intracerebral hemorrhage; Intracranial pressure; Stroke.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare no competing interests.
Figures
References
-
- Wilkinson CM, Kung TFC, Jickling GC, Colbourne F. A translational perspective on intracranial pressure responses following intracerebral hemorrhage in animal models. Brain Hemorrhages. 2021;2:34–48. doi: 10.1016/J.HEST.2020.10.002. - DOI
-
- Nadeau CA, Dietrich K, Wilkinson CM, Crawford AM, George GN, Nichol HK, et al. Prolonged blood-brain barrier injury occurs after experimental intracerebral hemorrhage and is not acutely associated with additional bleeding. Transl Stroke Res. 2019;10:287–297. doi: 10.1007/s12975-018-0636-9. - DOI - PMC - PubMed
-
- McDowell MM, Ducruet AF, Friedlander RM. Management of cerebral edema/intracranial pressure in ischemic stroke. Primer on cerebrovascular diseases: Academic Press; 2017. pp. 738–742.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
