Renal TLR-7/TNF-α pathway as a potential female-specific mechanism in the pathogenesis of autoimmune-induced hypertension
- PMID: 36367687
- PMCID: PMC9744658
- DOI: 10.1152/ajpheart.00286.2022
Renal TLR-7/TNF-α pathway as a potential female-specific mechanism in the pathogenesis of autoimmune-induced hypertension
Abstract
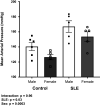
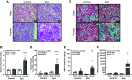
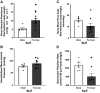
Hypertension is prevalent in patients with systemic lupus erythematosus (SLE). The goal of the current study is to track the pathogenesis of hypertension and renal injury in SLE, identify contributory mechanisms, and highlight differences in disease development among sexes. Mean arterial pressure was measured in conscious male and female SLE (NZBWF1) and control (NZW) mice at 34-35 wk of age using indwelling arterial catheters. Measures of renal injury, renal inflammation, and renal hemodynamics were used to monitor the potential contributors to latent sex differences. Both male and female SLE mice were hypertensive at 35 wk of age, and the hypertension was linked to renal injury in females, but not in males. A known contributor of renal pathology in SLE, Toll-like receptor (TLR)-7, and its downstream effector, the proinflammatory cytokine tumor necrosis factor (TNF)-α, were lower in male SLE mice than in females. Male SLE mice also had higher glomerular filtration rate (GFR) and lower renal vascular resistance (RVR) than females. Our data suggest that although hypertension in female SLE mice is associated with renal mechanisms, hypertension in male SLE mice may develop independent of renal changes. Future studies will continue to dissect sex-specific factors that should be considered when treating patients with hypertension with underlying chronic inflammation and/or autoimmunity.NEW & NOTEWORTHY There is a high prevalence of hypertension in male and female SLE; however, male SLE mice are hypertensive without renal involvement. The development of hypertension in female SLE mice is renocentric and strongly associated with injurious renal mechanisms like the TLR-7→TNF-α pathway. This clear difference in the pathogenesis among the sexes could have a significant impact on how we treat patients with hypertension with underlying chronic autoimmune/inflammatory diseases.
Keywords: Toll-like receptor 7; blood pressure; renal hemodynamics; renal injury; systemic lupus erythematosus.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Comment in
-
A song of AAs and fire: divergent sex-dependent renal inflammatory mechanisms in hypertensive SLE mice.Am J Physiol Heart Circ Physiol. 2023 Jan 1;324(1):H82-H84. doi: 10.1152/ajpheart.00679.2022. Epub 2022 Dec 9. Am J Physiol Heart Circ Physiol. 2023. PMID: 36487187 Free PMC article. No abstract available.
References
-
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 383: 1899–1911, 2014. doi: 10.1016/s0140-6736(14)60685-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

