SARS-CoV-2 infection downregulates myocardial ACE2 and potentiates cardiac inflammation in humans and hamsters
- PMID: 36367689
- PMCID: PMC9705018
- DOI: 10.1152/ajpheart.00578.2022
SARS-CoV-2 infection downregulates myocardial ACE2 and potentiates cardiac inflammation in humans and hamsters
Abstract
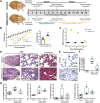
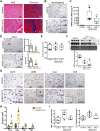
Myocardial pathologies resulting from SARS-CoV-2 infections are consistently rising with mounting case rates and reinfections; however, the precise global burden is largely unknown and will have an unprecedented impact. Understanding the mechanisms of COVID-19-mediated cardiac injury is essential toward the development of cardioprotective agents that are urgently needed. Assessing novel therapeutic strategies to tackle COVID-19 necessitates an animal model that recapitulates human disease. Here, we sought to compare SARS-CoV-2-infected animals with patients with COVID-19 to identify common mechanisms of cardiac injury. Two-month-old hamsters were infected with either the ancestral (D614) or Delta variant (B.1.617.2) of SARS-CoV-2 for 2 days, 7 days, and/or 14 days. We measured viral RNA and cytokine expression at the earlier time points to capture the initial stages of infection in the lung and heart. We assessed myocardial angiotensin-converting enzyme 2 (ACE2), the entry receptor for the SARS-CoV-2 virus, and cardioprotective enzyme, as well as markers for inflammatory cell infiltration in the hamster hearts at days 7 and 14. In parallel, human hearts were stained for ACE2, viral nucleocapsid, and inflammatory cells. Indeed, we identify myocardial ACE2 downregulation and myeloid cell burden as common events in both hamsters and humans infected with SARS-CoV-2, and we propose targeting downstream ACE2 downregulation as a therapeutic avenue that warrants clinical investigation.NEW & NOTEWORTHY Cardiac manifestations of COVID-19 in humans are mirrored in the SARS-CoV-2 hamster model, recapitulating myocardial damage, ACE2 downregulation, and a consistent pattern of immune cell infiltration independent of viral dose and variant. Therefore, the hamster model is a valid approach to study therapeutic strategies for COVID-19-related heart disease.
Keywords: ACE2; COVID-19; heart; inflammation.
Conflict of interest statement
Z. Kassiri is an editor of
Figures

References
-
- Wang K, Gheblawi M, Nikhanj A, Munan M, MacIntyre E, O'Neil C, Poglitsch M, Colombo D, Del Nonno F, Kassiri Z, Sligl W, Oudit GY. Dysregulation of ACE (angiotensin-converting enzyme)-2 and renin-angiotensin peptides in SARS-CoV-2 mediated mortality and end-organ injuries. Hypertension 79: 365–378, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18295. - DOI - PubMed
-
- Chan JF-W, Zhang AJ, Yuan S, Poon VK-M, Chan CC-S, Lee AC-Y, Chan W-M, Fan Z, Tsoi H-W, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai J-P, Kok K-H, Chu H, Chan K-H, Sridhar S, Chen Z, Chen H, To KK-W, Yuen K-Y. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 71: 2428–2446, 2020. doi: 10.1093/cid/ciaa325. - DOI - PMC - PubMed
-
- Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, Beermann ML, Simone-Roach C, Lindstrom-Vautrin J, Olejnik J, Suder EL, Bullitt E, Hinds A, Sharma A, Bosmann M, Wang R, Hawkins F, Burks EJ, Saeed M, Wilson AA, Mühlberger E, Kotton DN. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 27: 962–973.e7, 2020. doi: 10.1016/j.stem.2020.09.013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

