Structure-Activity Studies of 1 H-Imidazo[4,5- c]quinolin-4-amine Derivatives as A3 Adenosine Receptor Positive Allosteric Modulators
- PMID: 36367749
- PMCID: PMC10354740
- DOI: 10.1021/acs.jmedchem.2c01170
Structure-Activity Studies of 1 H-Imidazo[4,5- c]quinolin-4-amine Derivatives as A3 Adenosine Receptor Positive Allosteric Modulators
Abstract
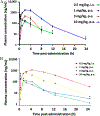
We previously reported 1H-imidazo[4,5-c]quinolin-4-amines as A3 adenosine receptor (A3AR) positive allosteric modulators (PAMs). A3AR agonists, but not PAMs, are in clinical trials for inflammatory diseases and liver conditions. We synthesized new analogues to distinguish 2-cyclopropyl antagonist 17 (orthosteric interaction demonstrated by binding and predicted computationally) from PAMs (derivatives with large 2-alkyl/cycloalkyl/bicycloalkyl groups). We predicted PAM binding at a hydrophobic site on the A3AR cytosolic interface. Although having low Caco-2 permeability and high plasma protein binding, hydrophobic 2-cyclohept-4-enyl-N-3,4-dichlorophenyl, MRS7788 18, and 2-heptan-4-yl-N-4-iodophenyl, MRS8054 39, derivatives were orally bioavailable in rat. 2-Heptan-4-yl-N-3,4-dichlorophenyl 14 and 2-cyclononyl-N-3,4-dichlorophenyl 20 derivatives and 39 greatly enhanced Cl-IB-MECA-stimulated [35S]GTPγS binding Emax, with only 12b trending toward decreasing the agonist EC50. A feasible route for radio-iodination at the p-position of a 4-phenylamino substituent suggests a potential radioligand for allosteric site binding. Herein, we advanced an allosteric approach to developing A3AR-activating drugs that are potentially event- and site-specific in action.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Gessi S; Merighi S; Varani K Adenosine receptors: The status of the art. In The Adenosine Receptors; Borea PA., Varani K., Gessi S., Merighi S., Vincenzi F., Eds.; Springer International Publishing, 2018; pp 1–11.
-
- Linden J Adenosine in tissue protection and tissue regeneration. Mol. Pharmacol. 2005, 67 (5), 1385. - PubMed
-
- Borea PA; Varani K; Vincenzi F; Baraldi PG; Tabrizi MA; Merighi S; Gessi S The A3 adenosine receptor: History and perspectives. Pharmacol. Rev. 2015, 67 (1), 74. - PubMed
-
- Changeux J-P; Edelstein SJ Allosteric mechanisms of signal transduction. Science 2005, 308 (5727), 1424–1428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous

