Phase Reconstruction of Low-Energy Electron Holograms of Individual Proteins
- PMID: 36367752
- PMCID: PMC9706659
- DOI: 10.1021/acsnano.2c06897
Phase Reconstruction of Low-Energy Electron Holograms of Individual Proteins
Abstract
Low-energy electron holography (LEEH) is one of the few techniques capable of imaging large and complex three-dimensional molecules, such as proteins, on the single-molecule level at subnanometer resolution. During the imaging process, the structural information about the object is recorded both in the amplitude and in the phase of the hologram. In low-energy electron holography imaging of proteins, the object's amplitude distribution, which directly reveals molecular size and shape on the single-molecule level, can be retrieved via a one-step reconstruction process. However, such a one-step reconstruction routine cannot directly recover the phase information encoded in the hologram. In order to extract the full information about the imaged molecules, we thus implemented an iterative phase retrieval algorithm and applied it to experimentally acquired low-energy electron holograms, reconstructing the phase shift induced by the protein along with the amplitude data. We show that phase imaging can map the projected atomic density of the molecule given by the number of atoms in the electron path. This directly implies a correlation between reconstructed phase shift and projected mean inner potential of the molecule, and thus a sensitivity to local changes in potential, an interpretation that is further substantiated by the strong phase signatures induced by localized charges.
Keywords: hologram reconstruction; low-energy electron holography; phase retrieval; protein imaging; single-molecule imaging.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

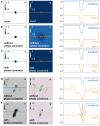
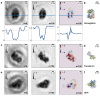

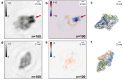
References
-
- Fink H.-W.; Schmid H.; Ermantraut E.; Schulz T. Electron Holography of Individual DNA Molecules. J. Opt. Soc. Am. A 1997, 14, 2168–2172. 10.1364/JOSAA.14.002168. - DOI
-
- Ochner H.; Szilagyi S.; Abb S.; Gault J.; Robinson C. V.; Malavolti L.; Rauschenbach S.; Kern K. Low-Energy Electron Holography Imaging of Conformational Variability of Single-Antibody Molecules from Electrospray Ion Beam Deposition. Proc. Natl. Acad. Sci. U. S. A 2021, 118 (51), e2112651118. 10.1073/pnas.2112651118. - DOI - PMC - PubMed
-
- Nakane T.; Kotecha A.; Sente A.; McMullan G.; Masiulis S.; Brown P. M. G. E.; Grigoras I. T.; Malinauskaite L.; Malinauskas T.; Miehling J.; Uchanski T.; Yu L.; Karia D.; Pechnikova E. V.; de Jong E.; Keizer J.; Bischoff M.; McCormack J.; Tiemeijer P.; Hardwick S. W.; et al. Single-Particle cryo-EM at Atomic Resolution. Nature 2020, 587, 152–156. 10.1038/s41586-020-2829-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

