Krill oil protects dopaminergic neurons from age-related degeneration through temporal transcriptome rewiring and suppression of several hallmarks of aging
- PMID: 36367773
- PMCID: PMC9699765
- DOI: 10.18632/aging.204375
Krill oil protects dopaminergic neurons from age-related degeneration through temporal transcriptome rewiring and suppression of several hallmarks of aging
Abstract
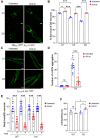
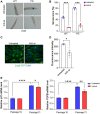
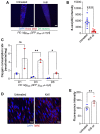
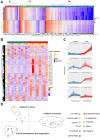
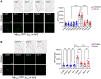
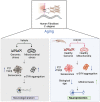
There is accumulating evidence that interfering with the basic aging mechanisms can enhance healthy longevity. The interventional/therapeutic strategies targeting multiple aging hallmarks could be more effective than targeting one hallmark. While health-promoting qualities of marine oils have been extensively studied, the underlying molecular mechanisms are not fully understood. Lipid extracts from Antarctic krill are rich in long-chain omega-3 fatty acids choline, and astaxanthin. Here, we used C. elegans and human cells to investigate whether krill oil promotes healthy aging. In a C. elegans model of Parkinson´s disease, we show that krill oil protects dopaminergic neurons from aging-related degeneration, decreases alpha-synuclein aggregation, and improves dopamine-dependent behavior and cognition. Krill oil rewires distinct gene expression programs that contribute to attenuating several aging hallmarks, including oxidative stress, proteotoxic stress, senescence, genomic instability, and mitochondrial dysfunction. Mechanistically, krill oil increases neuronal resilience through temporal transcriptome rewiring to promote anti-oxidative stress and anti-inflammation via healthspan regulating transcription factors such as SNK-1. Moreover, krill oil promotes dopaminergic neuron survival through regulation of synaptic transmission and neuronal functions via PBO-2 and RIM-1. Collectively, krill oil rewires global gene expression programs and promotes healthy aging via abrogating multiple aging hallmarks, suggesting directions for further pre-clinical and clinical explorations.
Keywords: aging; healthspan; krill oil; mitochondrial health; senescence.
Conflict of interest statement
Figures


References
-
- Fang EF, Xie C, Schenkel JA, Wu C, Long Q, Cui H, Aman Y, Frank J, Liao J, Zou H, Wang NY, Wu J, Liu X, et al.. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res Rev. 2020; 64:101174. 10.1016/j.arr.2020.101174 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

