Light triggers a network switch between circadian morning and evening oscillators controlling behaviour during daily temperature cycles
- PMID: 36367867
- PMCID: PMC9683589
- DOI: 10.1371/journal.pgen.1010487
Light triggers a network switch between circadian morning and evening oscillators controlling behaviour during daily temperature cycles
Abstract
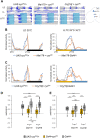
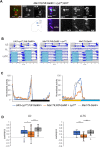
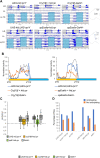
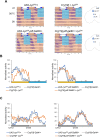
Proper timing of rhythmic locomotor behavior is the consequence of integrating environmental conditions and internal time dictated by the circadian clock. Rhythmic environmental input like daily light and temperature changes (called Zeitgeber) reset the molecular clock and entrain it to the environmental time zone the organism lives in. Furthermore, depending on the absolute temperature or light intensity, flies exhibit their main locomotor activity at different times of day, i.e., environmental input not only entrains the circadian clock but also determines the phase of a certain behavior. To understand how the brain clock can distinguish between (or integrate) an entraining Zeitgeber and environmental effects on activity phase, we attempted to entrain the clock with a Zeitgeber different from the environmental input used for phasing the behavior. 150 clock neurons in the Drosophila melanogaster brain control different aspects of the daily activity rhythms and are organized in various clusters. During regular 12 h light: 12 h dark cycles at constant mild temperature (LD 25°C, LD being the Zeitgeber), so called morning oscillator (MO) neurons control the increase of locomotor activity just before lights-on, while evening oscillator (EO) neurons regulate the activity increase at the end of the day, a few hours before lights-off. Here, using 12 h: 12 h 25°C:16°C temperature cycles as Zeitgeber, we attempted to look at the impact of light on phasing locomotor behavior. While in constant light and 25°C:16°C temperature cycles (LLTC), flies show an unimodal locomotor activity peak in the evening, during the same temperature cycle, but in the absence of light (DDTC), the phase of the activity peak is shifted to the morning. Here, we show that the EO is necessary for synchronized behavior in LLTC but not for entraining the molecular clock of the other clock neuronal groups, while the MO controls synchronized morning activity in DDTC. Interestingly, our data suggest that the influence of the EO on the synchronization increases depending on the length of the photoperiod (constant light vs 12 h of light). Hence, our results show that effects of different environmental cues on clock entrainment and activity phase can be separated, allowing to decipher their integration by the circadian clock.
Copyright: © 2022 Lorber et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

