AMPK-dependent phosphorylation of MTFR1L regulates mitochondrial morphology
- PMID: 36367943
- PMCID: PMC9651865
- DOI: 10.1126/sciadv.abo7956
AMPK-dependent phosphorylation of MTFR1L regulates mitochondrial morphology
Abstract
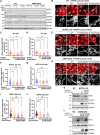
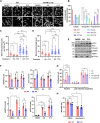
Mitochondria are dynamic organelles that undergo membrane remodeling events in response to metabolic alterations to generate an adequate mitochondrial network. Here, we investigated the function of mitochondrial fission regulator 1-like protein (MTFR1L), an uncharacterized protein that has been identified in phosphoproteomic screens as a potential AMP-activated protein kinase (AMPK) substrate. We showed that MTFR1L is an outer mitochondrial membrane-localized protein modulating mitochondrial morphology. Loss of MTFR1L led to mitochondrial elongation associated with increased mitochondrial fusion events and levels of the mitochondrial fusion protein, optic atrophy 1. Mechanistically, we show that MTFR1L is phosphorylated by AMPK, which thereby controls the function of MTFR1L in regulating mitochondrial morphology both in mammalian cell lines and in murine cortical neurons in vivo. Furthermore, we demonstrate that MTFR1L is required for stress-induced AMPK-dependent mitochondrial fragmentation. Together, these findings identify MTFR1L as a critical mitochondrial protein transducing AMPK-dependent metabolic changes through regulation of mitochondrial dynamics.
Figures




References
-
- Wai T., Langer T., Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 27, 105–117 (2016). - PubMed
-
- Tabara L. C., Morris J. L., Prudent J., The complex dance of organelles during mitochondrial division. Trends Cell Biol. 31, 241–253 (2021). - PubMed
-
- Kraus F., Roy K., Pucadyil T. J., Ryan M. T., Function and regulation of the divisome for mitochondrial fission. Nature 590, 57–66 (2021). - PubMed
-
- Giacomello M., Pyakurel A., Glytsou C., Scorrano L., The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

