Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells
- PMID: 36367947
- PMCID: PMC9847353
- DOI: 10.1126/sciimmunol.abq3254
Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells
Abstract
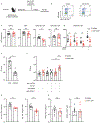
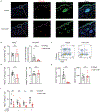
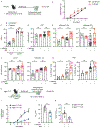
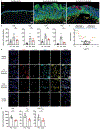
The cytokine interleukin-23 (IL-23) is critical for development and maintenance of autoimmune inflammation in nonlymphoid tissues; however, the mechanism through which IL-23 supports tissue-specific immunity remains unclear. In mice, we found that circulating memory T cells were dispensable for anamnestic protection from Candida albicans skin infection, and tissue-resident memory (TRM) cell-mediated protection from C. albicans reinfection required IL-23. Administration of anti-IL-23 receptor antibody to mice after resolution of primary C. albicans infection resulted in loss of CD69+ CD103+ tissue-resident memory T helper 17 (TRM17) cells from skin, and clinical anti-IL-23 therapy depleted TRM17 cells from skin of patients with psoriasis. IL-23 receptor blockade impaired TRM17 cell proliferation but did not affect apoptosis susceptibility or tissue egress. IL-23 produced by CD301b+ myeloid cells was required for TRM17 maintenance in skin after C. albicans infection, and CD301b+ cells were necessary for TRM17 expansion during the development of imiquimod dermatitis. This study demonstrates that locally produced IL-23 promotes in situ proliferation of cutaneous TRM17 cells to support their longevity and function and provides mechanistic insight into the durable efficacy of IL-23 blockade in the treatment of psoriasis.
Figures



References
-
- Hirai T, Yang Y, Zenke Y, Li H, Chaudhri VK, Diaz JSDLC, Zhou PY, Nguyen BA-T, Bartholin L, Workman CJ, Griggs DW, Vignali DAA, Singh H, Masopust D, Kaplan DH, Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche. Immunity. 54, 84–98.e5 (2021). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

