Potential Mediators of Oxaliplatin-Induced Peripheral Neuropathy From Adjuvant Therapy in Stage III Colon Cancer: Findings From CALGB (Alliance)/SWOG 80702
- PMID: 36367997
- PMCID: PMC9928634
- DOI: 10.1200/JCO.22.01637
Potential Mediators of Oxaliplatin-Induced Peripheral Neuropathy From Adjuvant Therapy in Stage III Colon Cancer: Findings From CALGB (Alliance)/SWOG 80702
Abstract
Purpose: We sought to evaluate the independent and interactive associations of planned treatment duration, celecoxib use, physical activity, body mass index (BMI), diabetes mellitus, and vitamin B6 with oxaliplatin-induced peripheral neuropathy (OIPN) among patients with stage III colon cancer enrolled in a clinical trial.
Methods: We conducted a prospective, observational study of 2,450 patients with stage III colon cancer enrolled in the CALGB/SWOG 80702 trial, randomly assigned to 6 versus 12 cycles of adjuvant fluorouracil, leucovorin, and oxaliplatin chemotherapy with or without 3 years of celecoxib. OIPN was reported using the Common Terminology Criteria for Adverse Events (CTCAE) during and following completion of chemotherapy and the FACT/GOG-NTX-13 15-17 months after random assignment. Multivariate analyses were adjusted for baseline sociodemographic and clinical factors.
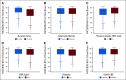
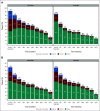
Results: Patients assigned to 12 treatment cycles, relative to 6, were significantly more likely to experience higher-grade CTCAE- and FACT/GOG-NTX-13-reported neuropathy and longer times to resolution, while neither celecoxib nor vitamin B6 intake attenuated OIPN. Exercising ≥ 9 MET-hours per week after treatment relative to < 9 was associated with improvements in FACT/GOG-NTX-13-reported OIPN (adjusted difference in means, 1.47; 95% CI, 0.49 to 2.45; P = .003). Compared with patients with baseline BMIs < 25, those with BMIs ≥ 25 were at significantly greater risk of developing higher-grade CTCAE-reported OIPN during (adjusted odds ratio, 1.18; 95% CI, 1.00 to 1.40; P = .05) and following completion (adjusted odds ratio, 1.23; 95% CI, 1.01 to 1.50; P = .04) of oxaliplatin treatment. Patients with diabetes were significantly more likely to experience worse FACT/GOG-NTX-13-reported neuropathy relative to those without (adjusted difference in means, -2.0; 95% CI, -3.3 to -0.73; P = .002). There were no significant interactions between oxaliplatin treatment duration and any of these potentially modifiable exposures.
Conclusion: Lower physical activity, higher BMI, diabetes, and longer planned treatment duration, but not celecoxib use or vitamin B6 intake, may be associated with significantly increased OIPN severity.
Trial registration: ClinicalTrials.gov NCT01150045.
Conflict of interest statement
Potential Mediators of Oxaliplatin-Induced Peripheral Neuropathy from Adjuvant Therapy in Stage III Colon Cancer: Findings from CALGB (Alliance)/SWOG 80702
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Pulvers JN, Marx G. Factors associated with the development and severity of oxaliplatin-induced peripheral neuropathy: A systematic review. Asia Pac J Clin Oncol. 2017;13:345–355. - PubMed
-
- Hsu SY, Huang WS, Lee SH, et al. Incidence, severity, longitudinal trends and predictors of acute and chronic oxaliplatin-induced peripheral neuropathy in Taiwanese patients with colorectal cancer. Eur J Cancer Care (Engl) 2019;28:e12976. - PubMed
-
- Yamaguchi K, Kusaba H, Makiyama A, et al. The risk factors for oxaliplatin-induced peripheral sensory neuropathy and thrombocytopenia in advanced gastric cancer. Cancer Chemother Pharmacol. 2018;82:625–633. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233320/CA/NCI NIH HHS/United States
- UG1 CA233234/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA189954/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233163/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233337/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

