Salinity-induced ionoregulatory changes in the gill proteome of the mayfly, Neocloeon triangulifer
- PMID: 36368556
- PMCID: PMC12184953
- DOI: 10.1016/j.envpol.2022.120609
Salinity-induced ionoregulatory changes in the gill proteome of the mayfly, Neocloeon triangulifer
Abstract
Ecologists have observed declines in the biodiversity of sensitive freshwater organisms in response to increasing concentrations of major ions (salinization). Yet, how changing salinities physiologically challenge aquatic organisms, such as mayflies, remains remarkably understudied. Moreover, it is not well understood the degree to which species respond and acclimate to salinity changes. Our lab is developing the Baetid mayfly, N. triangulifer, as a model organism for physiological research. We have previously described acclimatory changes in both ion flux rates and altered mRNA transcript levels in response to chronic exposures to elevated major ion concentrations at the whole animal level. In the present study, we use shotgun proteomics to identify the specific proteins associated with apical ion transport and how their abundance changes in response to chronic salinity exposures in gills. Gills were isolated from the penultimate nymphal stage of N. triangulifer reared under control culture conditions, elevated NaCl (157 mg L-1 Na), elevated CaCl2 (121 mg L-1 Ca), elevated Ca/MgSO4 (735 mg L-1 SO4). These conditions mirrored those from previously published physiological work. We also acutely exposed nymphs to dilute (50% dilution of culture water with deionized water) to explore proteomic changes in the gills in response to dilute conditions. We report 710 unique peptide sequences among treatment groups, including important apical ion transporters such as Ca-ATPase, Na/K ATPase, and V-ATPase. Treatment with elevated NaCl and Ca/MgSO4 appeared to cause more significant differential protein expression (452 and 345, respectively) compared to CaCl2 and dilute groups (134 and 17, respectively). Finally, we demonstrated the breadth of physiological functions in gills by exploring non-transport related pathways found in our dataset, including ATP synthesis, calcium signaling, and oxidative stress response. We discuss our results in the context of freshwater salinization and the challenges of working with non-model species without fully sequenced and annotated genomes.
Keywords: Gill biology; Ion transport; Mayfly; Osmoregulation; Proteomics; Salinity.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

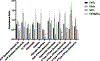

References
-
- Berrill M, Taylor G, Savard H, 1991. Are chloride cells involved in low pH tolerance and sensitivity? The mayfly possibility. Can. J. Fish. Aquat. Sci. 48, 1220–1225. 10.1139/f91-147. - DOI
-
- Bradley TJ, Stuart AM, Satir P, 1982. The ultrastructure of the larval malpighian tubules of a saline-water mosquito. Tissue Cell 14, 759–773. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

