Plasticity of perisynaptic astroglia during ischemia-induced spreading depolarization
- PMID: 36368909
- PMCID: PMC10152098
- DOI: 10.1093/cercor/bhac434
Plasticity of perisynaptic astroglia during ischemia-induced spreading depolarization
Abstract
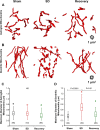
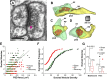
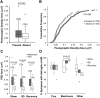
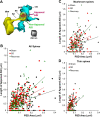
High astroglial capacity for glutamate and potassium clearance aids in recovering spreading depolarization (SD)-evoked disturbance of ion homeostasis during stroke. Since perisynaptic astroglia cannot be imaged with diffraction-limited light microscopy, nothing is known about the impact of SD on the ultrastructure of a tripartite synapse. We used serial section electron microscopy to assess astroglial synaptic coverage in the sensorimotor cortex of urethane-anesthetized male and female mice during and after SD evoked by transient bilateral common carotid artery occlusion. At the subcellular level, astroglial mitochondria were remarkably resilient to SD compared to dendritic mitochondria that were fragmented by SD. Overall, 482 synapses in `Sham' during `SD' and `Recovery' groups were randomly selected and analyzed in 3D. Perisynaptic astroglia was present at the axon-spine interface (ASI) during SD and after recovery. Astrocytic processes were more likely found at large synapses on mushroom spines after recovery, while the length of the ASI perimeter surrounded by astroglia has also significantly increased at large synapses. These findings suggest that as larger synapses have a bigger capacity for neurotransmitter release during SD, they attract astroglial processes to their perimeter during recovery, limiting extrasynaptic glutamate escape and further enhancing the astrocytic ability to protect synapses in stroke.
Keywords: astrocyte; mitochondria; quantitative serial section electron microscopy; stroke; tripartite synapse.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta. 2008:1777:953–964. - PubMed
-
- Aizawa H, Sun W, Sugiyama K, Itou Y, Aida T, Cui W, Toyoda S, Terai H, Yanagisawa M, Tanaka K. Glial glutamate transporter GLT-1 determines susceptibility to spreading depression in the mouse cerebral cortex. Glia. 2020:68:2631–2642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

