Surgical results of the Lung Cancer Mutation Consortium 3 trial: A phase II multicenter single-arm study to investigate the efficacy and safety of atezolizumab as neoadjuvant therapy in patients with stages IB-select IIIB resectable non-small cell lung cancer
- PMID: 36369159
- PMCID: PMC10288861
- DOI: 10.1016/j.jtcvs.2022.10.007
Surgical results of the Lung Cancer Mutation Consortium 3 trial: A phase II multicenter single-arm study to investigate the efficacy and safety of atezolizumab as neoadjuvant therapy in patients with stages IB-select IIIB resectable non-small cell lung cancer
Abstract
Objective: Multimodality treatment for resectable non-small cell lung cancer has long remained at a therapeutic plateau. Immune checkpoint inhibitors are highly effective in advanced non-small cell lung cancer and promising preoperatively in small clinical trials for resectable non-small cell lung cancer. This large multicenter trial tested the safety and efficacy of neoadjuvant atezolizumab and surgery.
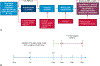
Methods: Patients with stage IB to select IIIB resectable non-small cell lung cancer and Eastern Cooperative Oncology Group performance status 0/1 were eligible. Patients received atezolizumab 1200 mg intravenously every 3 weeks for 2 cycles or less followed by resection. The primary end point was major pathological response in patients without EGFR/ALK+ alterations. Pre- and post-treatment computed tomography, positron emission tomography, pulmonary function tests, and biospecimens were obtained. Adverse events were recorded by Common Terminology Criteria for Adverse Events v.4.0.
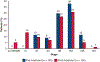
Results: From April 2017 to February 2020, 181 patients were entered in the study. Baseline characteristics were mean age, 65.1 years; female, 93 of 181 (51%); nonsquamous histology, 112 of 181 (62%); and clinical stages IIB to IIIB, 147 of 181 (81%). In patients without EGFR/ALK alterations who underwent surgery, the major pathological response rate was 20% (29/143; 95% confidence interval, 14-28) and the pathological complete response rate was 6% (8/143; 95% confidence interval, 2-11). There were no grade 4/5 treatment-related adverse events preoperatively. Of 159 patients (87.8%) undergoing surgery, 145 (91%) had pathologic complete resection. There were 5 (3%) intraoperative complications, no intraoperative deaths, and 2 postoperative deaths within 90 days, 1 treatment related. Median disease-free and overall survival have not been reached.
Conclusions: Neoadjuvant atezolizumab in resectable stage IB to IIIB non-small cell lung cancer was well tolerated, yielded a 20% major pathological response rate, and allowed safe, complete surgical resection. These results strongly support the further development of immune checkpoint inhibitors as preoperative therapy in locally advanced non-small cell lung cancer.
Keywords: immunotherapy; lung cancer; neoadjuvant therapy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
V.W.R. is a member of the Data Safety and Monitoring Committee for the MARS2 trial (UK), serves as Co-Chair of the National Cancer Institute Thoracic Staging Malignancy Committee, and reports institutional funding from Genentech. A.N. is an employee of Genentech and reports stock ownership with Roche. S.N.W. reports research support grants from AbbVie Inc, Ariad Pharmaceuticals, Genentech, ImmunoMedics, Inc, Millennium Pharmaceuticals Inc, Roche, Astellas Pharma Inc, Daiichi Sankyo, Cullinan Pearl, Verastem Inc, GlaxoSmithKline/GSK, Janssen Research & Development, LLC, Elevation Oncology, Daiichi Sankyo, Genentech, Loxo Oncology, Takeda Pharmaceuticals Company Limited, grant from the SWOG Clinical Trials Partnership, honorarium from ASCO, and Chair of Data Safety Monitoring Board for the Hoosier Cancer Research Network. E.M.T. reports honoraria from Intuitive Surgical. E.B.H. serves in a consulting or advisory role to Amgen, Ellipses Pharma, Janssen Oncology, Janssen Research & Development, and Revolution Medicines; reports research funding (paid to his institution) from AstraZeneca, Genentech, Incyte, Janssen, Novartis, Revolution Medicines, and Spectrum Pharmaceuticals; and reports patents, royalties, or other intellectual property from Protein-Protein Interactions as Biomarkers Patent. K.L.R. reports personal fees from Amgen, Calithera, AstraZeneca, Blueprint, Boehringer Ingelheim, Daiichi Sankyo, EMD Soreno, Genentech, GlaxoSmithKline, Janssen, Lilly, Merck KGA, Mirati, Takeda, Tesaro, and nonfinancial support from Seattle Genetics; research support to institution from CALITHERA, Blueprint, Daiichi Sankyo, Genentech, Elevation Oncology, and Janssen outside the submitted work. R.E.M. reports speaker fees from Intuitive Surgical. D.H.O. reports funding to his institution from Genentech, Merck, Pfizer, Palobiofarma, and Bristol Myers Squibb. E.B.G. reports grant and research support from ABL Bio, AstraZeneca, Bristol Myers Squibb, Dynavax Technologies, EMD Serono, Genentech, Iovance Biotherapeutics, Eli Lilly & Co, Merck, Mirati Therapeutics, Neon Therapeutics, and Novartis; and consulting or advisory roles with ABL Bio, Boehringer Ingelheim, Bristol Myers Squibb, Dracen Pharmaceuticals, Eisai, Eli Lilly, EMD Serono, Gilead, GSK, Merck, Natera, Novartis, Personalis, Regeneron, Sanofi, Shionogi, and Xilio Therapeutics. R.C.D. is an employee and shareholder of Rain Therapeutics and has received consulting fees, travel reimbursement, and licensing fees from Genentech/Roche; and has received funding from the National Institutes of Health/National Cancer Institute University of Colorado Lung SPORE (5P50CA058187). M.N. reports honoraria from Astra Zeneca (ad-board), Caris Life Sciences (consultant), Lilly (consultant), Daiichi Sankyo (ad-board), Takeda (speaker), Novartis (ad-board), EMD Serono (ad-board), Blueprint Medicines (speaker), Janssen (ad-board), Pfizer (ad-board), Genentech (ad-board), and has received travel support from AnHeart Therapeutics. H.I.P. is a member of the Genentech Steering Committee for IMPOWER030 and the SKYSCRAPER Trial. K.S. is an employee of Genentech and reports stock ownership with Roche. A.J. is an employee of Genentech and reports stock ownership with Roche. P.A.B. is on Advisory or Data Safety Monitoring Boards for BMS and Merck, and reports membership on the Board of Directors for Verastem, Inc., with funding to his institution. B.E.J. reports consultant fees from Checkpoint Therapeutics, Genentech, Hummingbird Diagnostics and Hengrui Therapeutics. M.G.K. reports speaking fees from AstraZeneca and Pfizer, consultant fees for Janssen, and in-kind support for medical writing from Hoffman-La Roche. D.J.K. serves as an advisor to AstraZeneca and Genentech/Roche and reports research funding from AADi. I.I.W. reports grants and personal fees from Genentech/Roche, Bayer, Bristol-Myers Squibb, AstraZeneca, Pfizer, HTG Molecular, GlaxoSmithKline, Guardant Health, Merck, Novartis, Sanofi, and Amgen; personal fees from Asuragen, Flame, Daiichi Sankyo, Oncocyte, MSD, and Platform Health; grants from Adaptive, Adaptimmune, EMD Serono, Takeda, Karus, Johnson & Johnson, 4D, Iovance, and Akoya, outside the submitted work. J.E.C. serves as an advisor to Genentech/Roche, AstraZeneca/MedImmune, Merck, Bristol Myers Squibb, Flame Biosciences, Janssen Oncology, Guardant Health, Regeneron/Sanofi, and Novartis; and reports research funding from Genentech/Roche, Bristol Myers Squibb, AstraZeneca/MedImmune, and Merck. D.P.C. serves in a consultant or advisory role to AbbVie, Agenus, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, EMD Serono, Genentech/Roche, Helsinn Healthcare, Incyte, Inivata, Inovio Pharmaceuticals, Janssen, Kyowa Hakko Kirin, Merck, Novartis, Pfizer, prIME Oncology, and Takeda, and research funding from Bristol Myers Squibb and Genentech. J.M.L. reports grants, consulting fees, and honoraria from AstraZeneca, BMS, Genentech, and Novartis, and leadership roles at AstraZeneca, Genentech, and Novartis. All other authors reported no conflicts of interest.
The
Figures

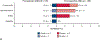
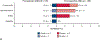
Comment in
-
Commentary: Neoadjuvant immune checkpoint monotherapy for lung cancer: Has the train left the station?J Thorac Cardiovasc Surg. 2023 Mar;165(3):840-841. doi: 10.1016/j.jtcvs.2022.10.031. Epub 2022 Oct 30. J Thorac Cardiovasc Surg. 2023. PMID: 36402582 No abstract available.
References
-
- Goldstraw P, Crowley J, Rami-Porta R, Rusch VW, Postmus PE, The IASLC Prognostic Factors Committee, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the eighth edition of the TNM classification of malignant tumors. J Thorac Oncol. 2015;11:39–51.
-
- Pless M, Stupp R, Ris H-B, Stahel RA, Weder W, Thierstein S, et al. Induction chemoradiation in stage IIIA/N2 non-small cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386:1049–56. - PubMed
-
- Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT III, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small cell lung cancer: mature results of Southwest Oncology Group Phase II study 8805. J Clin Oncol. 1995;13:1880–92. - PubMed
-
- Van Meerbeeck JP, Kramer GWPM, Van Schil PEY, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Nat Cancer Inst. 2007;99:442–50. - PubMed
-
- Pisters KMW, Vallières E, Crowley JJ, Franklin WA, Bunn PA Jr, Ginsberg RJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early stage non-small cell lung cancer: Southwest Oncology Group trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28:1843–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

