The neuroimmunology of social-stress-induced sensitization
- PMID: 36369271
- PMCID: PMC10000282
- DOI: 10.1038/s41590-022-01321-z
The neuroimmunology of social-stress-induced sensitization
Abstract
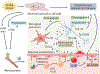
Myriad clinical findings provide links between chronic stressors, inflammation, and mood disorders. Furthermore, traumatic or chronic exposure to psychological stressors may promote stress sensitization, in which individuals have long-term complications, including increased vulnerability to subsequent stressors. Post-traumatic stress disorder (PTSD) is a clinically relevant example of stress sensitization. PTSD alters neuronal circuitry and mood; however, the mechanisms underlying long-term stress sensitization within this disorder are unclear. Rodent models of chronic social defeat recapitulate several key physiological, immunological, and behavioral responses associated with psychological stress in humans. Repeated social defeat (RSD) uniquely promotes the convergence of neuronal, central inflammatory (microglial), and peripheral immune (monocyte) pathways, leading to prolonged anxiety, social withdrawal, and cognitive impairment. Moreover, RSD promotes stress sensitization, in which mice are highly sensitive to subthreshold stress exposure and recurrence of anxiety weeks after the cessation of stress. Therefore, the purpose of this Review is to discuss the influence of social-defeat stress on the immune system that may underlie stress sensitization within three key cellular compartments: neurons, microglia, and monocytes. Delineating the mechanisms of stress sensitization is critical in understanding and treating conditions such as PTSD.
© 2022. Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



References
-
- Pace TW & Heim CM A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav. Immun. 25, 6–13 (2012). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

