Prediction of Busulfan Clearance by Predose Plasma Metabolomic Profiling
- PMID: 36369996
- PMCID: PMC9888309
- DOI: 10.1002/cpt.2794
Prediction of Busulfan Clearance by Predose Plasma Metabolomic Profiling
Abstract
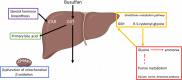
Intravenous busulfan doses are often personalized to a target plasma exposure (targeted busulfan) using an individual's busulfan clearance (BuCL). We evaluated whether BuCL could be predicted by a predose plasma panel of 841 endogenous metabolomic compounds (EMCs). In this prospective cohort of 132 hematopoietic cell transplantation (HCT) patients, all had samples collected immediately before busulfan administration (preBU) and 96 had samples collected 2 weeks before busulfan (2-week-preBU). BuCL was significantly associated with 37 EMCs after univariate linear regression analysis and controlling for false discovery (< 0.05) in the 132 preBU samples. In parallel, with preBU samples, we included all 841 EMCs in a least absolute shrinkage and selection operator-penalized regression which selected 13 EMCs as predominantly associated with BuCL. Then, we constructed a prediction model by estimating coefficients for these 13 EMCs, along with sex, using ordinary least-squares. When the resulting linear prediction model was applied to the 2-week-preBU samples, it explained 40% of the variation in BuCL (adjusted R2 = 0.40). Pathway enrichment analysis revealed 18 pathways associated with BuCL. Lysine degradation followed by steroid biosynthesis, which aligned with the univariate analysis, were the top two pathways. BuCL can be predicted before busulfan administration with a linear regression model of 13 EMCs. This pharmacometabolomics method should be prioritized over use of a busulfan test dose or pharmacogenomics to guide busulfan dosing. These results highlight the potential of pharmacometabolomics as a precision medicine tool to improve or replace pharmacokinetics to personalize busulfan doses.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

Comment in
-
Could Metabolomics Be the Key to Unlocking Precision Dosing in the Clinic?Clin Pharmacol Ther. 2023 Feb;113(2):207-209. doi: 10.1002/cpt.2811. Clin Pharmacol Ther. 2023. PMID: 36693112 No abstract available.
References
-
- Palmer, J. et al. Personalizing busulfan‐based conditioning: considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol. Blood Marrow Transplant. 22, 1915–1925 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

